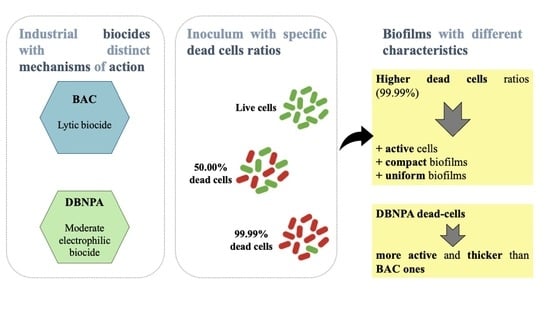
Influence of Dead Cells Killed by Industrial Biocides (BAC and DBNPA) on Biofilm Formation
Abstract
:1. Introduction
2. Results
2.1. The Impact of Dead Cells on Biofilm Formation
2.2. Biofilm Thickness and Structure
2.3. EPS Quantification
3. Discussion
3.1. How Does the Planktonic Cells’ Killing Method Affect the Biofilm Properties?
3.2. The Effects of Inoculum with Different Dead/Live Cell Ratios on Biofilm Build-Up
4. Materials and Methods
4.1. Biocides
4.2. Microorganisms and Culturing Conditions
4.3. Obtaining Dead Bacterial Cells
4.4. Biofilm Formation on a PPFC System
4.5. Biofilm Analysis
4.5.1. Biofilm Visualisation Using Optical Coherence Tomography
4.5.2. Sampling
4.5.3. Culturability
4.5.4. Cell Membrane Integrity
4.5.5. Cellular Energy
4.5.6. Metabolic Activity
4.5.7. EPS Extraction
Protein Quantification
Polysaccharide Quantification
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maillard, J.-Y. Antimicrobial biocides in the healthcare environment: Efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar]
- Russell, A. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. 2002, 92, 121S–135S. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.; Catrenich, C.; Charbonneau, D.; Bartolo, R. Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. J. Hosp. Infect. 2003, 55, 98–107. [Google Scholar] [CrossRef]
- Barros, A.C.; Melo, L.F.; Pereira, A. A Multi-purpose approach to the mechanisms of action of two biocides (benzalkonium chloride and dibromonitrilopropionamide): Discussion of Pseudomonas fluorescens’ viability and death. Front. Microbiol. 2022, 13, 842414. [Google Scholar] [CrossRef]
- Barros, A.C.; Melo, L.F.; Pereira, A. Pseudomonas fluorescens cells’ recovery after exposure to BAC and DBNPA biocides. Antibiotics 2022, 11, 1042. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Walker, D.M.; Harshey, R.M. Dead cells release a ‘necrosignal’that activates antibiotic survival pathways in bacterial swarms. Nat. Commun. 2020, 11, 4157. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, M.; Kirkpatrick, R.L.; Montauti, E.I.; Tran, B.Q.; Peterson, S.B.; Harding, B.N.; Whitney, J.C.; Russell, A.B.; Traxler, B.; Goo, Y.A. Kin cell lysis is a danger signal that activates antibacterial pathways of Pseudomonas aeruginosa. eLife 2015, 4, e05701. [Google Scholar] [CrossRef] [PubMed]
- Maffei, E.; Harms, A. Messages from the dead protect bacteria from viral attack. EMBO J. 2022, 41, e110382. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Land, W.G.; Paczesny, S. Danger signals triggering immune response and inflammation. Front. Immunol. 2017, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Smakman, F. Effects of Dead Bacterial Cells on Growth, Gene Expression and Evolution of Surrounding Microbes. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2021. [Google Scholar]
- Chatzigiannidou, I.; Props, R.; Boon, N. Drinking water bacterial communities exhibit specific and selective necrotrophic growth. NPJ Clean Water 2018, 1, 22. [Google Scholar] [CrossRef]
- Smakman, F.; Hall, A.R. Exposure to lysed bacteria can promote or inhibit growth of neighboring live bacteria depending on local abiotic conditions. FEMS Microbiol. Ecol. 2022, 98, fiac011. [Google Scholar] [CrossRef] [PubMed]
- Zayed, N.; Figueiredo, J.; Van Holm, W.; Boon, N.; Bernaerts, K.; Teughels, W. Mode of killing determines the necrotrophic response of oral bacteria. J. Oral Microbiol. 2023, 15, 2184930. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Sarrafzadeh, M.H.; Mehrnia, M.R. Fouling in membrane bioreactors with various concentrations of dead cells. Desalination 2011, 278, 373–380. [Google Scholar] [CrossRef]
- Herzberg, M.; Elimelech, M. Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 2007, 295, 11–20. [Google Scholar] [CrossRef]
- Kim, L.H.; Shin, M.S.; Kim, S.-J.; Kim, C.-M.; Chae, K.-J.; Kim, I.S. Potential effects of damaged Pseudomonas aeruginosa PAO1 cells on development of reverse osmosis membrane biofouling. J. Membr. Sci. 2015, 477, 86–92. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, L.H.; Kim, S.-J.; Kim, C.-M.; Chae, K.-J.; Kim, I.S. Effect of dead cells on biofouling in the reverse osmosis process. Water Supply 2013, 13, 1396–1401. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Hou, L.-a. Impact of dead cells on biofouling and pharmaceutically active compounds retention by NF/RO membranes. Chem. Eng. J. 2018, 337, 51–59. [Google Scholar] [CrossRef]
- Narciso, D.A.; Pereira, A.; Dias, N.O.; Melo, L.F.; Martins, F.G. Characterization of biofilm structure and properties via processing of 2D optical coherence tomography images in BISCAP. Bioinformatics 2022, 38, 1708–1715. [Google Scholar] [CrossRef]
- Bajpai, P. The control of microbiological problems. In Pulp and Paper Industry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 103–195. [Google Scholar]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Kucera, J. Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Pinel, I.; Prest, E.; Bucs, S.; Van Loosdrecht, M.; Kruithof, J.; Vrouwenvelder, J.S. Application of DBNPA dosage for biofouling control in spiral wound membrane systems. Desalination Water Treat. 2017, 68, 12–22. [Google Scholar] [CrossRef]
- Akhova, A.V.; Tkachenko, A.G. ATP/ADP alteration as a sign of the oxidative stress development in Escherichia coli cells under antibiotic treatment. FEMS Microbiol. Lett. 2014, 353, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Brown, D.G. Variation in bacterial ATP level and proton motive force due to adhesion to a solid surface. Appl. Environ. Microbiol. 2009, 75, 2346–2353. [Google Scholar] [CrossRef]
- Schink, S.J.; Biselli, E.; Ammar, C.; Gerland, U. Death rate of E. coli during starvation is set by maintenance cost and biomass recycling. Cell Syst. 2019, 9, 64–73. [Google Scholar] [CrossRef]
- Robinson, T.P.; Aboaba, O.O.; Kaloti, A.; Ocio, M.J.; Baranyi, J.; Mackey, B.M. The effect of inoculum size on the lag phase of Listeria monocytogenes. Int. J. Food Microbiol. 2001, 70, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, R.; Vervaeren, H.; Noseda, B.; Boon, N.; Verstraete, W. Necrotrophic growth of Legionella pneumophila. Appl. Environ. Microbiol. 2006, 72, 4323–4328. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, D.; Veenendaal, H.R.; Scheffer, W.J. Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res. 2005, 39, 2789–2798. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J.A.; Hiraoka, K. Microbe-surface interactions in biofouling and biocorrosion processes. Int. Microbiol. 2005, 8, 157–168. [Google Scholar] [PubMed]
- Takano, S.; Pawlowska, B.J.; Gudelj, I.; Yomo, T.; Tsuru, S. Density-dependent recycling promotes the long-term survival of bacterial populations during periods of starvation. MBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Goller, C.C.; Romeo, T. Environmental Influences on Biofilm Development. In Bacterial Biofilms; Romeo, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 37–66. [Google Scholar] [CrossRef]
- Ferreira, C.; Rosmaninho, R.; Simões, M.; Pereira, M.; Bastos, M.; Nunes, O.; Coelho, M.; Melo, L. Biofouling control using microparticles carrying a biocide. Biofouling 2009, 26, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Araújo, J.; Miranda, J.; Simões, M.; Melo, L.; Mergulhão, F. The effects of surface properties on Escherichia coli adhesion are modulated by shear stress. Colloids Surf. B Biointerfaces 2014, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bakke, R.; Kommedal, R.; Kalvenes, S. Quantification of biofilm accumulation by an optical approach. J. Microbiol. Methods 2001, 44, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.; Reed, G. “Drop plate” method of counting viable bacteria. Can. J. Res. 1948, 26, 317–326. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Simões, M.; Pereira, M.O.; Vieira, M. The role of hydrodynamic stress on the phenotypic characteristics of single and binary biofilms of Pseudomonas fluorescens. Water Sci. Technol. 2007, 55, 437–445. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, A.C.; Narciso, D.A.C.; Melo, L.F.; Pereira, A. Influence of Dead Cells Killed by Industrial Biocides (BAC and DBNPA) on Biofilm Formation. Antibiotics 2024, 13, 140. https://doi.org/10.3390/antibiotics13020140
Barros AC, Narciso DAC, Melo LF, Pereira A. Influence of Dead Cells Killed by Industrial Biocides (BAC and DBNPA) on Biofilm Formation. Antibiotics. 2024; 13(2):140. https://doi.org/10.3390/antibiotics13020140
Chicago/Turabian StyleBarros, Ana C., Diogo A. C. Narciso, Luis F. Melo, and Ana Pereira. 2024. "Influence of Dead Cells Killed by Industrial Biocides (BAC and DBNPA) on Biofilm Formation" Antibiotics 13, no. 2: 140. https://doi.org/10.3390/antibiotics13020140