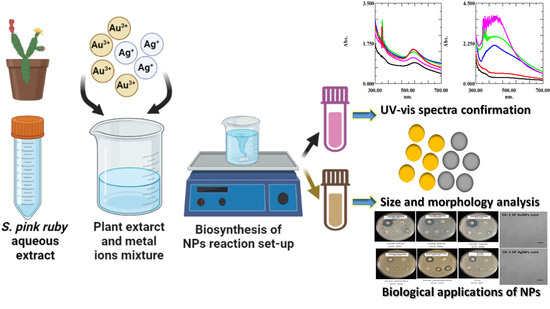
Sedeveria pink ruby Extract-Mediated Synthesis of Gold and Silver Nanoparticles and Their Bioactivity against Livestock Pathogens and in Different Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV–Visible (UV–Vis) Spectrophotometry of SP-AuNPs and SP-AgNPs
2.2. FESEM-EDX Characterization of SP-AuNPs and SP-AgNPs
2.3. Transmission Electron Microscopy (TEM) of SP-AuNPs and SP-AgNPs
2.4. AFM Characterization of SP-AuNPs and SP-AgNPs
2.5. FT-IR Spectroscopic Analysis of SP-AuNPs and SP-AgNPs
2.6. Powder XRD Characterization of SP-AuNPs and SP-AgNPs
2.7. Antibacterial Activity of SP-AuNPs and SP-AgNPs
2.8. Antifungal Activity of SP-AuNPs and SP-AgNPs
2.9. Antioxidant Activity of SP-AuNPs and SP-AgNPs
2.10. Cytotoxicity of SP-AuNPs and SP-AgNPs
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Plant Collection and Preparation of Aqueous Extract
4.3. Biosynthesis of AuNPs and AgNPs
4.4. Characterization of SP-AuNPs and SP-AgNPs
4.5. Antibacterial Activity of SP-AuNPs and SP-AgNPs
4.6. Antifungal Activity of SP-AuNPs and SP-AgNPs
4.7. MIC Study
4.8. Antioxidant Activity of SP-AuNPs and SP-AgNPs
4.9. Cytotoxicity of SP-AuNPs and SP-AgNPs
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.Y.; Jang, H.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Amjad, R.; Mubeen, B.; Ali, S.S.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Rasool, R.; Ullah, I.; Nadeem, M.S.; et al. Green synthesis and characterization of copper nanoparticles using Fortunella margarita Leaves. Polymers 2021, 13, 4364. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, N. Silver Nanoparticles: Synthesis, Characterization and Applications. In Silver Nanoparticles: Fabrication, Characterization and Applications; Maaz, K., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Aabed, K.; Mohammed, A.E. Phytoproduct, arabic gum and Opophytum forsskalii seeds for bio-fabrication of silver nanoparticles: Antimicrobial and cytotoxic capabilities. Nanomaterials 2021, 11, 2573. [Google Scholar] [CrossRef]
- Banu, H.; Renuka, N.; Faheem, S.M.; Ismail, R.; Singh, V.; Saadatmand, Z.; Khan, S.S.; Narayanan, K.; Raheem, A.; Premkumar, K.; et al. Gold and Silver Nanoparticles Biomimetically Synthesized Using Date Palm Pollen Extract-Induce Apoptosis and Regulate p53 and Bcl-2 Expression in Human Breast Adenocarcinoma Cells. Biol. Trace Elem. Res. 2018, 186, 122–134. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadagouda, M.N.; Iyanna, N.; Lalley, J.; Han, C.; Dionysiou, D.D.; Varma, R.S. Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate, and turmeric extracts. ACS Sustain. Chem. Eng. 2014, 2, 1717–1723. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, J.O.; Seo, Y.B.; Kang, M.J.; Kim, G.D. Biogenic Synthesis of Metallic Nanoparticles and Their Antibacterial Applications Biogenic Synthesis of Metallic Nanoparticles and Their Antibacterial Applications. J. Life Sci. 2021, 31, 862–872. [Google Scholar]
- Da Silva, A.L.; Santos, R.S.; Xisto, D.G.; Alonso, S.D.V.; Morales, M.M.; Rocco, P.R.M. Nanoparticle-based therapy for respiratory diseases. An. Acad. Bras. Ciências 2013, 85, 137–146. [Google Scholar] [CrossRef]
- Flores-Lopez, N.S.; Cervantes-Chávez, J.A.; Téllez de Jesús, D.G.; Cortez-Valadez, M.; Estévez-González, M.; Esparza, R. Bactericidal and fungicidal capacity of Ag2O/Ag nanoparticles synthesized with Aloe vera extract. J. Environ. Sci. Health Part A 2021, 56, 762–768. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.O.; Zamudio-Ojeda, A.; León-Morales, J.M.; López-Velázquez, J.C.; García-Morales, S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J. Sci. Food Agric. 2021, 101, 1270–1287. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Ek, E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 6, 439–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Wampande, E.M.; Ejobi, F.; Nakavuma, J.L.; Maaza, M.; Sackey, J.; Nxumalo, E.; Kirabira, J.B. Green Strategy–Based Synthesis of Silver Nanoparticles for Antibacterial Applications. Front. Nanotechnol. 2021, 3, 697303. [Google Scholar] [CrossRef]
- Kumari, M.M.; Philip, D. Facile one-pot synthesis of gold and silver nanocatalysts using edible coconut oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 154–160. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Ali, N.; Lin, Y.-C.; Karmenyan, A.; Chang, C.-C.; Bibikova, O.; Skovorodkin, I.; Prunskaite-Hyyryläinen, R.; Vainio, S.J.; Kinnunen, M.; et al. Au nanostar nanoparticle as a bio-imaging agent and its detection and visualization in biosystems. Biomed. Opt. Express 2020, 11, 5872. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; De La Zerda, A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Somu, P.; Parida, U.K.; Mishra, G. Apoptotic Effect and Anticancer Activity of Biosynthesized Silver Nanoparticles from Marine Algae Chaetomorpha linum Extract against Human Colon Cancer Cell HCT-116. Biol. Trace Elem. Res. 2021, 199, 1812–1822. [Google Scholar] [CrossRef]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl. Mater. Interfaces 2009, 1, 1553–1560. [Google Scholar] [CrossRef]
- Hussain, J.I.; Kumar, S.; Hashmi, A.A.; Khan, Z. Silver nanoparticles: Preparation, characterization, and kinetics. Int. Assoc. Adv. Mater. 2011, 2, 188–194. [Google Scholar]
- Kapoor, S.; Sood, H.; Saxena, S.; Chaurasia, O.P. Green synthesis of silver nanoparticles using Rhodiola imbricata and Withania somnifera root extract and their potential catalytic, antioxidant, cytotoxic and growth-promoting activities. Bioprocess Biosyst. Eng. 2022, 45, 365–380. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Dou, D.; Ge, L.; Huang, Z.; Wang, L.; Gu, N. A caffeic acid mediated facile synthesis of silver nanoparticles with powerful anti-cancer activity. Colloids Surf. B Biointerfaces 2015, 134, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Pulit, J.; Banach, M. Preparation of nanosilver and nanogold based on dog rose aqueous extract. Bioinorg. Chem. Appl. 2014, 2014, 658935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-mediated biosynthesis of nanoparticles: Applications and future prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Din, M.I.; Bashir, S.; Qadir, M.A.; Rashid, F. Green Synthesis and Characterization of Silver Nanoparticles Using Ferocactus echidne Extract as a Reducing Agent. Anal. Lett. 2015, 48, 1180–1189. [Google Scholar] [CrossRef]
- Huo, Y.; Singh, P.; Kim, Y.J.; Soshnikova, V.; Kang, J.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Mathiyalagan, R.; Chokkalingam, M.; et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Adewale, O.B.; Egbeyemi, K.A.; Onwuelu, J.O.; Potts-Johnson, S.S.; Anadozie, S.O.; Fadaka, A.O.; Osukoya, O.A.; Aluko, B.T.; Johnson, J.; Obafemi, T.O.; et al. Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon 2020, 6, e05501. [Google Scholar] [CrossRef]
- Chahardoli, A.; Hajmomeni, P.; Ghowsi, M.; Qalekhani, F.; Shokoohinia, Y.; Fattahi, A. Optimization of quercetin-assisted silver nanoparticles synthesis and evaluation of their hemocompatibility, antioxidant, anti-inflammatory, and antibacterial effects. Glob. Chall. 2021, 5, 2100075. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Lee, C.S. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Fu, L.H.; Yang, J.; Zhu, J.F.; Ma, M.G. Synthesis of Gold Nanoparticles and Their Applications in Drug Delivery. In Metal Nanoparticles in Pharma; Springer: Berlin/Heidelberg, Germany, 2017; pp. 155–191. [Google Scholar]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac. J. Trop. Biomed. 2012, 2, 574–580. [Google Scholar]
- Kharey, P.; Dutta, S.B.; Gorey, A.; Manikandan, M.; Kumari, A.; Vasudevan, S.; Palani, I.A.; Majumder, S.K.; Gupta, S. Pimenta dioica Mediated Biosynthesis of Gold Nanoparticles and Evaluation of Its Potential for Theranostic Applications. ChemistrySelect 2020, 5, 7901–7908. [Google Scholar] [CrossRef]
- Doan, V.D.; Luc, V.S.; Nguyen, T.L.H.; Nguyen, T.D.; Nguyen, T.D. Utilizing waste corn-cob in biosynthesis of noble metallic nanoparticles for antibacterial effect and catalytic degradation of contaminants. Environ. Sci. Pollut. Res. 2020, 27, 6148–6162. [Google Scholar] [CrossRef]
- Kang, J.P.; Kim, Y.J.; Singh, P.; Huo, Y.; Soshnikova, V.; Markus, J.; Ahn, S.; Chokkalingam, M.; Lee, H.A.; Yang, D.C. Biosynthesis of gold and silver chloride nanoparticles mediated by Crataegus pinnatifida fruit extract: In Vitro study of anti-inflammatory activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1530–1540. [Google Scholar] [CrossRef] [Green Version]
- Valsalam, S.; Agastian, P.; Esmail, G.A.; Ghilan, A.K.M.; Al-Dhabi, N.A.; Arasu, M.V. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol. B Biol. 2019, 201, 111670. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Qu, D.; Sun, W.; Chen, Y.; Zhou, J.; Liu, C. Synthesis and in vitro antineoplastic evaluation of silver nanoparticles mediated by Agrimoniae herba extract. Int. J. Nanomed. 2014, 9, 1871–1882. [Google Scholar]
- Le, V.T.; Ngu, N.N.Q.; Chau, T.P.; Nguyen, T.D.; Nguyen, V.T.; Nguyen, T.L.H.; Cao, X.T.; Doan, V.D. Silver and Gold Nanoparticles from Limnophila rugosa Leaves: Biosynthesis, Characterization, and Catalytic Activity in Reduction of Nitrophenols. J. Nanomater. 2021, 2021, 5571663. [Google Scholar] [CrossRef]
- Francis, S.; Koshy, E.P.; Mathew, B. Green synthesis of Stereospermum suaveolens capped silver and gold nanoparticles and assessment of their innate antioxidant, antimicrobial and antiproliferative activities. Bioprocess Biosyst. Eng. 2018, 41, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.; Singh, T.; Kumar, A. Aqueous-mixed ionic liquid system: Phase transitions and synthesis of gold nanocrystals. Langmuir 2011, 27, 9261–9269. [Google Scholar] [CrossRef] [PubMed]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Sci. Iran. 2013, 20, 1049–1054. [Google Scholar]
- Terletskaya, N.V.; Seitimova, G.A.; Kudrina, N.O.; Meduntseva, N.D.; Ashimuly, K. The Reactions of Photosynthetic Capacity and Plant Metabolites of Sedum hybridum L. in Response to Mild and Moderate Abiotic Stresses. Plants 2022, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Avila, P.E.; Patakfalvi, R.; Rodríguez-Pedroza, C.; Aparicio-Fernández, X.; Loza-Cornejo, S.; Villa-Cruz, V.; Martínez-Cano, E. One-pot green synthesis of gold and silver nanoparticles using: Rosa canina L. extract. RSC Adv. 2021, 11, 14624–14631. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, H.; Ahn, S.; Castro-Aceituno, V.; Jiménez, Z.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Pharmacological importance, characterization and applications of gold and silver nanoparticles synthesized by Panax ginseng fresh leaves. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B. Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Jahan, I.; Erci, F.; Isildak, I. Rapid green synthesis of non-cytotoxic silver nanoparticles using aqueous extracts of ‘Golden Delicious’ apple pulp and cumin seeds with antibacterial and antioxidant activity. SN Appl. Sci. 2021, 3, 94. [Google Scholar] [CrossRef]
- Alwhibi, M.S.; Soliman, D.A.; Awad, M.A.; Alangery, A.B.; Al Dehaish, H.; Alwasel, Y.A. Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications. Green Process. Synth. 2021, 10, 412–420. [Google Scholar] [CrossRef]
- Christopher, J.S.G.; Saswati, B.; Ezilrani, P.S. Optimization of parameters for biosynthesis of silver nanoparticles using leaf extract of Aegle marmelos. Braz. Arch. Biol. Technol. 2015, 58, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E.; Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E.; Banti, C.N.; Rossos, A.K. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Rahaman Mollick, M.M.; Bhowmick, B.; Mondal, D.; Maity, D.; Rana, D.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Sarkar, J.; Acharya, K.; et al. Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route. RSC Adv. 2014, 4, 37838–37848. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Nikaeen, G.; Yousefinejad, S.; Rahmdel, S.; Samari, F.; Mahdavinia, S. Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity. Sci. Rep. 2020, 10, 9642. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of silver nanoparticles by Aspergillus terreus: Characterization, optimization, and biological activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef]
- Shah, M.Z.; Guan, Z.H.; Din, A.U.; Ali, A.; Rehman, A.U.; Jan, K.; Faisal, S.; Saud, S.; Adnan, M.; Wahid, F.; et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. 2021, 111, 20754. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of Gold Nanoparticles Using Mimosa tenuiflora Extract, Assessments of Cytotoxicity, Cellular Uptake, and Catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Mathiyalagan, R.; Kim, Y.J.; Castro-Aceituno, V.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int. J. Nanomed. 2016, 11, 3691–3701. [Google Scholar]
- Suliman, Y.A.O.; Ali, D.; Alarifi, S.; Harrath, A.H.; Mansour, L.; Alwasel, S.H. Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic effect of silver nanoparticles in human lung epithelial cells. Environ. Toxicol. 2015, 30, 149–160. [Google Scholar] [CrossRef]
- Delalat, R.; Sadat Shandiz, S.A.; Pakpour, B. Antineoplastic effectiveness of silver nanoparticles synthesized from Onopordum acanthium L. extract (AgNPs-OAL) toward MDA-MB231 breast cancer cells. Mol. Biol. Rep. 2022, 49, 1113–1120. [Google Scholar] [CrossRef]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloid Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Majumdar, M.; Biswas, S.C.; Choudhury, R.; Upadhyay, P.; Adhikary, A.; Roy, D.N.; Misra, T.K. Synthesis of Gold Nanoparticles Using Citrus macroptera Fruit Extract: Anti-Biofilm and Anticancer Activity. ChemistrySelect 2019, 4, 5714–5723. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laban, B.; Ralević, U.; Petrović, S.; Leskovac, A.; Vasić-Anićijević, D.; Marković, M.; Vasić, V. Green synthesis and characterization of nontoxic L-methionine capped silver and gold nanoparticles. J. Inorg. Biochem. 2020, 204, 110958. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Saipriya, K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012, 7, 1189–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapolisi, A.M.; Nkanga, C.I.; Walker, R.B.; Krause, R.W.M. Simultaneous liposomal encapsulation of antibiotics and proteins: Co-loading and characterization of rifampicin and Human Serum Albumin in soy-liposomes. J. Drug Deliv. Sci. Technol. 2020, 58, 101751. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int. J. Pharm. 2009, 526, 466–473. [Google Scholar] [CrossRef]
- Zia, F.; Ghafoor, N.; Iqbal, M.; Mehboob, S. Green synthesis and characterization of silver nanoparticles using Cydonia oblong seed extract. Appl. Nanosci. 2016, 6, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wei, Y.; Fang, C.; Chen, J.; Zhao, W. Facile synthesis of CQDs/Ag NPs composites with photoluminescence and their potential application in antibacterial materials. Inorg. Chem. Commun. 2021, 134, 109059. [Google Scholar] [CrossRef]
- Fafal, T.; Taştan, P.; Tüzün, B.S.; Ozyazici, M.; Kivcak, B. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Asphodelus aestivus Brot. aerial part extract. S. Afr. J. Bot. 2017, 112, 346–353. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Bhagat, E.; Sadras, S.R.; Nakkala, J.R.; Mata, R.; Bhagat, E.; Sadras, S.R. Green synthesis of silver and gold nanoparticles from Gymnema sylvestre leaf extract: Study of antioxidant and anticancer activities. J. Nanopart. Res. 2015, 17, 151. [Google Scholar] [CrossRef]







| Sr. No | Components | Antibacterial Activity in the Zone of Inhibition (mm) | Antifungal Activity | Antioxidant Activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | SE | SD | EC | YE | YS | CD | CA | CT | CG | DPPH (%) | ||
| 1 | SP extract | − | − | − | − | − | − | − | − | − | − | 38 |
| 2 | SP-AuNPs | + | − | + | w | w | +++ | ++ | + | + | − | 41 |
| 3 | SP-AgNPs | + | + | + | + | + | ++ | − | − | − | − | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppusamy, P.; Kim, S.; Kim, S.-J.; Park, M.; Song, K.-D. Sedeveria pink ruby Extract-Mediated Synthesis of Gold and Silver Nanoparticles and Their Bioactivity against Livestock Pathogens and in Different Cell Lines. Antibiotics 2023, 12, 507. https://doi.org/10.3390/antibiotics12030507
Kuppusamy P, Kim S, Kim S-J, Park M, Song K-D. Sedeveria pink ruby Extract-Mediated Synthesis of Gold and Silver Nanoparticles and Their Bioactivity against Livestock Pathogens and in Different Cell Lines. Antibiotics. 2023; 12(3):507. https://doi.org/10.3390/antibiotics12030507
Chicago/Turabian StyleKuppusamy, Palaniselvam, Sujung Kim, Sung-Jo Kim, Myunghum Park, and Ki-Duk Song. 2023. "Sedeveria pink ruby Extract-Mediated Synthesis of Gold and Silver Nanoparticles and Their Bioactivity against Livestock Pathogens and in Different Cell Lines" Antibiotics 12, no. 3: 507. https://doi.org/10.3390/antibiotics12030507