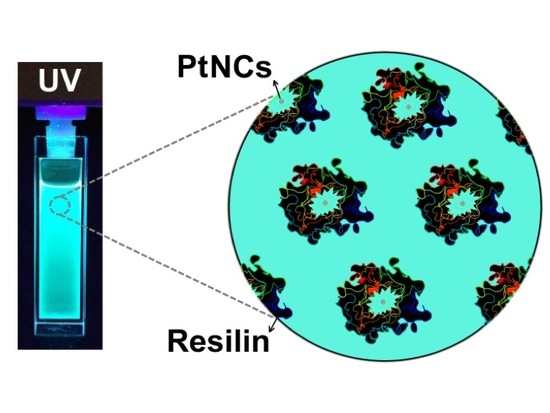
A Sustainable Biomineralization Approach for the Synthesis of Highly Fluorescent Ultra-Small Pt Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. Molecular Weight Determination by Matrix Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry
2.3. Synthesis of Fluorescent Pt-NMQCs
2.4. Photophysical Properties, Concentration, and Oxidation State of Pt-NMQCs
2.5. Small Angle X-ray Scattering (SAXS)
3. Results and Discussion
3.1. Photophysical Properties and Oxidation State of Pt-NMQCs
3.2. Inter-Dot Distance Dependence of the Fluorescence Property of Blue Fluorescent Pt-NMQCs-Rec1-Resilin Nanobioconjugates
3.3. Change in Conformational Organization of Rec1-Resilin in Pt-NMQC-Rec1-Resilin Nanobioconjugates
3.4. Evolution of Nucleated Pt-NMQCs with Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haberland, H. Clusters of Atoms and Molecules: Theory, Experiment, and Clusters of Atoms; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Halder, A.; Curtiss, L.A.; Fortunelli, A.; Vajda, S. Perspective: Size selected clusters for catalysis and electrochemistry. J. Chem. Phys. 2018, 148, 110901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajda, S.; White, M.G. Catalysis applications of size-selected cluster deposition. ACS Catal. 2015, 5, 7152–7176. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, C.; Dickson, R.M. Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys. Rev. Lett. 2004, 93, 077402. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly fluorescent noble-metal quantum dots. Ann. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef]
- Liu, M.; Tang, F.; Yang, Z.; Xu, J.; Yang, X. Recent progress on gold-nanocluster-based fluorescent probe for environmental analysis and biological sensing. J. Anal. Methods Chem. 2019, 2019, 1095148. [Google Scholar] [CrossRef]
- Li, D.; Chen, Z.; Mei, X. Fluorescence enhancement for noble metal nanoclusters. Adv. Colloid Interface Sci. 2017, 250, 25–39. [Google Scholar] [CrossRef]
- Khandelwal, P.; Poddar, P. Fluorescent metal quantum clusters: An updated overview of the synthesis, properties, and biological applications. J. Mater. Chem. B 2017, 5, 9055–9084. [Google Scholar] [CrossRef]
- Dutta, N.K.; Dutta, A.K.; Roy Choudhury, N. Gold nanoparticles and fluorescent proteins, optically coupled hybrid architectures. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 895–908. [Google Scholar]
- George, A.; Gopalakrishnan, H.; Mandal, S. Surfactant free platinum nanocluster as fluorescent probe for the selective detection of Fe (III) ions in aqueous medium. Sens. Actuators B 2017, 243, 332–337. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Su, H.; Chen, K.-J.; Armijo, A.L.; Lin, W.-Y.; Wang, Y.; Sun, J.; Kamei, K.-I.; Czernin, J.; et al. A supramolecular approach for preparation of size-controlled nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 4344–4348. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, S.; Albericio, F.; Kros, A. Amphiphilic peptides and their cross-disciplinary role as building blocks for nanoscience. Chem. Soc. Rev. 2010, 39, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Lad, A.N.; Singh, S.P.; Shanker, R. DNA assembled metal nanoclusters: Synthesis to novel applications. RSC Adv. 2016, 6, 113095–113114. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Goswami, N.; Giri, A.; Bootharaju, M.S.; Xavier, P.L.; Pradeep, T.; Pal, S.K. Copper quantum clusters in protein matrix: Potential sensor of pb2+ ion. Anal. Chem. 2011, 83, 9676–9680. [Google Scholar] [CrossRef]
- Yu, C.-J.; Chen, T.-H.; Jiang, J.-Y.; Tseng, W.-L. Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale 2014, 6, 9618–9624. [Google Scholar] [CrossRef]
- Balu, R.; Bourgeois, L.; Elvin, C.M.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. A multi-responsive intrinsically disordered protein (IDP)-directed green synthesis of fluorescent gold nanoclusters. J. Mater. Chem. B 2015, 3, 6580–6586. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Chang, J.; Zhang, B. Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 401–417. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, J.Y.; Wang, D.I.C. Uncovering the design rules for peptide synthesis of metal nanoparticles. J. Am. Chem. Soc. 2010, 132, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A. Protein-mediated biotemplating on the nanoscale. Biomimetics 2017, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Xavier, P.L.; Pradeep, T. Understanding the evolution of luminescent gold quantum clusters in protein templates. ACS Nano 2011, 5, 8816–8827. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.-J.; Xu, K. Fluorescent metal nanoclusters: From synthesis to applications. TrAC Trends Anal. Chem. 2014, 58, 90–98. [Google Scholar] [CrossRef]
- Boskey, A.L.; Villarreal-Ramirez, E. Intrinsically disordered proteins and biomineralization. Matrix Biol. 2016, 52–54, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.C.; Merritt, D.J.; Dixon, N.E. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef]
- Dutta, N.K.; Truong, M.Y.; Mayavan, S.; RoyChoudhury, N.; Elvin, C.M.; Kim, M.; Knott, R.; Nairn, K.M.; Hill, A.J. A genetically engineered protein responsive to multiple stimuli. Angew. Chem. Int. Ed. 2011, 50, 4428–4431. [Google Scholar] [CrossRef]
- Balu, R.; Whittaker, J.; Dutta, N.K.; Elvin, C.M.; Choudhury, N.R. Multi-responsive biomaterials and nanobioconjugates from resilin-like protein polymers. J. Mater. Chem. B 2014, 2, 5936–5947. [Google Scholar] [CrossRef]
- Mayavan, S.; Dutta, N.K.; Choudhury, N.R.; Kim, M.; Elvin, C.M.; Hill, A.J. Self-organization, interfacial interaction and photophysical properties of gold nanoparticle complexes derived from resilin-mimetic fluorescent protein rec1-resilin. Biomaterials 2011, 32, 2786–2796. [Google Scholar] [CrossRef]
- Dutta, N.K.; Choudhury, N.R.; Mayavan, S.; Balu, R.; Whittaker, J.; Elvin, C.M.; Hill, A.J. Template Directed Formation of Metal Nanoparticles and Uses Thereof. Patent WO2014071463A1, 15 May 2014. [Google Scholar]
- Balu, R.; Choudhury, N.R.; Mata, J.P.; de Campo, L.; Rehm, C.; Hill, A.J.; Dutta, N.K. Evolution of the interfacial structure of a catalyst ink with the quality of the dispersing solvent: A contrast variation small-angle and ultrasmall-angle neutron scattering investigation. ACS Appl. Mater. Interfaces 2019, 11, 9934–9946. [Google Scholar] [CrossRef] [PubMed]
- Konarev, P.V.; Volkov, V.V.; Sokolova, A.V.; Koch, M.H.J.; Svergun, D.I. Primus: A Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- Lipfert, J.; Doniach, S. Small-angle X-ray scattering from RNA, proteins, and protein complexes. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 307–327. [Google Scholar] [CrossRef] [PubMed]
- Svergun, D.I.; Koch, M.H.J.; Timmins, P.A.; May, R.P. Small Angle X-ray and Neutron Scattering from Solutions of Biological Macromolecules; OUP: Oxford, UK, 2013. [Google Scholar]
- Putnam, C.D.; Hammel, M.; Hura, G.L.; Tainer, J.A. X-ray solution scattering (SAXS) combined with crystallography and computation: Defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 2007, 40, 191–285. [Google Scholar] [CrossRef] [PubMed]
- Harms, G.S.; Pauls, S.W.; Hedstrom, J.F.; Johnson, C.K. Fluorescence and rotational dynamics of dityrosine. J. Fluoresc. 1997, 7, 283–292. [Google Scholar] [CrossRef]
- Atwood, C.S.; Perry, G.; Zeng, H.; Kato, Y.; Jones, W.D.; Ling, K.-Q.; Huang, X.; Moir, R.D.; Wang, D.; Sayre, L.M.; et al. Copper mediates dityrosine cross-linking of alzheimer’s amyloid-β. Biochemistry 2004, 43, 560–568. [Google Scholar] [CrossRef]
- Kawasaki, H.; Yamamoto, H.; Fujimori, H.; Arakawa, R.; Inada, M.; Iwasaki, Y. Surfactant-free solution synthesis of fluorescent platinum subnanoclusters. Chem. Commum. 2010, 46, 3759–3761. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, W.; Fayet, P.; Cox, D.M.; Fu, Z.; Kaldor, A.; Sherwood, R.; Sondericker, D. Photoemission from mass-selected monodispersed pt clusters. Phys. Rev. Lett. 1990, 64, 780–783. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, D.; Sacher, E. X-ray photoelectron spectroscopic analysis of Pt nanoparticles on highly oriented pyrolytic graphite, using symmetric component line shapes. J. Phys. Chem. C 2007, 111, 565–570. [Google Scholar] [CrossRef]
- Duchesne, P.N.; Zhang, P. Local structure of fluorescent platinum nanoclusters. Nanoscale 2012, 4, 4199–4205. [Google Scholar] [CrossRef]
- Tai, K.; Lü, W.; Umezu, I.; Sugimura, A. Inter-dot distance dependence of photoluminescence properties in cdse quantum dot systems. Appl. Phys. Express 2010, 3, 035202. [Google Scholar] [CrossRef]
- Balu, R.; Knott, R.; Cowieson, N.P.; Elvin, C.M.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. Structural ensembles reveal intrinsic disorder for the multi-stimuli responsive bio-mimetic protein rec1-resilin. Sci. Rep. 2015, 5, 10896. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Mata, J.P.; Knott, R.; Elvin, C.M.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. Effects of crowding and environment on the evolution of conformational ensembles of the multi-stimuli-responsive intrinsically disordered protein, rec1-resilin: A small-angle scattering investigation. J. Phys. Chem. B 2016, 120, 6490–6503. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, Z.; Yu, H.; Gong, Y.; Tan, Y.; Du, R.; Chen, W.; Xing, X.; Mo, G.; Chen, Z.; et al. Time-resolved small-angle X-ray scattering study on the growth behavior of silver nanoparticles. J. Phys. Chem. C 2014, 118, 11454–11463. [Google Scholar] [CrossRef]
- Koch, M.H.J.; Vachette, P.; Svergun, D.I. Small-angle scattering: A view on the properties, structures and structural changes of biological macromolecules in solution. Q. Rev. Biophys. 2003, 36, 147–227. [Google Scholar] [CrossRef] [PubMed]
- Bernadó, P. Effect of interdomain dynamics on the structure determination of modular proteins by small-angle scattering. Eur. Biophys. J. 2010, 39, 769–780. [Google Scholar] [CrossRef]
- Hammouda, B. Sans from homogeneous polymer mixtures: A unified overview. In Polymer Characteristics; Springer: Berlin/Heidelberg, Germany, 1993; pp. 87–133. [Google Scholar]
- Svergun, D.I.; Petoukhov, M.V.; Koch, M.H.J. Determination of domain structure of proteins from X-ray solution scattering. Biophys. J. 2001, 80, 2946–2953. [Google Scholar] [CrossRef]
- Wardle, B. Principles and Applications of Photochemistry; Wiley: Cornwall, UK, 2009. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balu, R.; Knott, R.; Elvin, C.M.; Hill, A.J.; R. Choudhury, N.; Dutta, N.K. A Sustainable Biomineralization Approach for the Synthesis of Highly Fluorescent Ultra-Small Pt Nanoclusters. Biosensors 2019, 9, 128. https://doi.org/10.3390/bios9040128
Balu R, Knott R, Elvin CM, Hill AJ, R. Choudhury N, Dutta NK. A Sustainable Biomineralization Approach for the Synthesis of Highly Fluorescent Ultra-Small Pt Nanoclusters. Biosensors. 2019; 9(4):128. https://doi.org/10.3390/bios9040128
Chicago/Turabian StyleBalu, Rajkamal, Robert Knott, Christopher M. Elvin, Anita J. Hill, Namita R. Choudhury, and Naba K. Dutta. 2019. "A Sustainable Biomineralization Approach for the Synthesis of Highly Fluorescent Ultra-Small Pt Nanoclusters" Biosensors 9, no. 4: 128. https://doi.org/10.3390/bios9040128