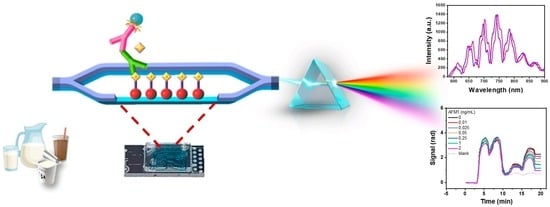
Mach-Zehnder Interferometric Immunosensor for Detection of Aflatoxin M1 in Milk, Chocolate Milk, and Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chip Fabrication and Instrumentation
2.3. Chip Activation
2.4. Preparation of Calibrators/Samples
2.5. AFM1 Detection with the MZI Immunosensor
3. Results and Discussion
3.1. Optimization of Assay Parameters
3.2. Matrix Effect of Milk, Chocolate Milk and Yogurt
3.3. Analytical Characteristics
3.4. Sensor Regeneration and Reuse
3.5. Comparison with Literature Optical Immunosensors for AFM1 Detection in Milk and Dairy Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Neal, G.E.; Eaton, D.L.; Judah, D.J.; Verma, A. Metabolism and toxicity of aflatoxins M1 and B1 in human-derived in vitro systems. Toxicol. Appl. Pharmacol. 1998, 151, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Veldman, A.; Meijs, J.A.C.; Borggreve, G.J.; Heeres-Van Der Tol, J.J. Carry-over of aflatoxin from cows’ food to milk. Anim. Sci. 1992, 55, 163–168. [Google Scholar] [CrossRef]
- Fedlu, M.; Seid, A.; Wollelie, A.; Mama, A. Aflatoxin and its Public Health Significance: A Review. Dairy Vet. Sci. J. 2019, 12, 555837. [Google Scholar] [CrossRef]
- IARC Monographs on the evaluation of carcinogenic risks to human. In Some Traditional Herbal Medicine, Some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; Volume 82.
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- EC (European Comission) Commission Regulation (EU) No 165/2010 of 26 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union. 2010, 50, 8–12.
- Fallah, A.A.; Rahnama, M.; Jafari, T.; Saei-Dehkordi, S.S. Seasonal variation of aflatoxin M1 contamination in industrial and traditional Iranian dairy products. Food Control 2011, 22, 1653–1656. [Google Scholar] [CrossRef]
- USFDA. CPG Sec. 527.400 Whole Milk, Low Fat Milk, Skim Milk-Aflatoxin M1 (CPG7106.210); FDA Compliance Policy Guides. United States Food and Drug Administration: Silver Spring, MD, USA, 1996.
- Vaz, A.; Cabral Silva, A.C.; Rodrigues, P.; Venâncio, A. Detection methods for aflatoxin m1 in dairy products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef]
- Shundo, L.; Sabino, M. Aflatoxin M1 in milk by immunoaffinity column cleanup with TLC/HPLC determination. Braz. J. Microbiol. 2006, 37, 164–167. [Google Scholar] [CrossRef]
- Pérez, E.; Martínez-Peinado, P.; Marco, F.; Gras, L.; Sempere, J.M.; Mora, J.; Grindlay, G. Determination of aflatoxin M1 in milk samples by means of an inductively coupled plasma mass spectrometry-based immunoassay. Food Chem. 2017, 230, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Maggira, M.; Ioannidou, M.; Sakaridis, I.; Samouris, G. Determination of aflatoxin m1 in raw milk using an HPLC-FL method in comparison with commercial elisa kits—Application in raw milk samples from various regions of Greece. Vet. Sci. 2021, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.E.; Gill, B.D.; Indyk, H.E.; Rhemrev, R.; Pazdanska, M.; Mackay, N.; Marley, E. Determination of Aflatoxin M1 in liquid milk, cheese, and selected milk proteins by automated online immunoaffinity cleanup with liquid chromatography—Fluorescence detection. J. AOAC Int. 2021, 104, 719–724. [Google Scholar] [CrossRef]
- Kos, J.; Hajnal, E.J.; Jajić, I.; Krstović, S.; Mastilović, J.; Šarić, B.; Jovanov, P. Comparison of ELISA, HPLC-FLD and HPLC-MS/MS methods for determination of aflatoxin M1 in natural contaminated milk samples. Acta Chim. Slov. 2016, 63, 747–756. [Google Scholar] [CrossRef]
- Tarannum, N.; Nipa, M.N.; Das, S.; Parveen, S. Aflatoxin M1 detection by ELISA in raw and processed milk in Bangladesh. Toxicol. Rep. 2020, 7, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiong, S.; Zhang, K.K.; Feng, L.; Chen, X.; Wu, Y.; Huang, X.; Xiong, Y. Quantum bead-based fluorescence-linked immunosorbent assay for ultrasensitive detection of aflatoxin M1 in pasteurized milk, yogurt, and milk powder. J. Dairy Sci. 2019, 102, 3985–3993. [Google Scholar] [CrossRef]
- Vdovenko, M.M.; Lu, C.C.; Yu, F.Y.; Sakharov, I.Y. Development of ultrasensitive direct chemiluminescent enzyme immunoassay for determination of aflatoxin M1 in milk. Food Chem. 2014, 158, 310–314. [Google Scholar] [CrossRef]
- Liu, B.H.; Chu, K.C.; Yu, F.Y. Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Thurner, F.; AlZahra’a Alatraktchi, F. Recent advances in electrochemical biosensing of aflatoxin M1 in milk—A mini review. Microchem. J. 2023, 190, 108594. [Google Scholar] [CrossRef]
- Abera, B.D.; Falco, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Development of flexible dispense-printed electrochemical immunosensor for aflatoxin m1 detection in milk. Sensors 2019, 19, 3912. [Google Scholar] [CrossRef]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.H. Rapid and sensitive inhibition-based assay for the electrochemical detection of Ochratoxin A and Aflatoxin M1 in red wine and milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- Tang, X.; Catanante, G.; Huang, X.; Marty, J.L.; Wang, H.; Zhang, Q.; Li, P. Screen-printed electrochemical immunosensor based on a novel nanobody for analyzing aflatoxin M1 in milk. Food Chem. 2022, 383, 132598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Meng, M.; Li, W.; Xiong, Y.; Fang, Y.; Lin, Q. Emerging biosensors to detect aflatoxin M1 in milk and dairy products. Food Chem. 2023, 398, 133848. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Dubiak-Szepietowska, M.; Vorobii, M.; Rodriguez-Emmenegger, C.; Dostálek, J.; Feller, K.-H. Sensitive and rapid detection of aflatoxin M1 in milk utilizing enhanced SPR and p(HEMA) brushes. Biosens. Bioelectron. 2016, 81, 159–165. [Google Scholar] [CrossRef]
- Chalyan, T.; Potrich, C.; Schreuder, E.; Falke, F.; Pasquardini, L.; Pederzolli, C.; Heideman, R.; Pavesi, L. AFM1 detection in milk by fab’ functionalized Si3N4 asymmetric mach-Zehnder interferometric biosensors. Toxins 2019, 11, 409. [Google Scholar] [CrossRef]
- Chalyan, T.; Guider, R.; Pasquardini, L.; Zanetti, M.; Falke, F.; Schreuder, E.; Heideman, R.G.; Pederzolli, C.; Pavesi, L. Asymmetric Mach-Zehnder interferometer based biosensors for Aflatoxin M1 detection. Biosensors 2016, 6, 1. [Google Scholar] [CrossRef]
- Indyk, H.E.; Chetikam, S.; Gill, B.D.; Wood, J.E.; Woollard, D.C. Development and application of an optical biosensor immunoassay for Aflatoxin M1 in bovine milk. Food Anal. Methods 2019, 12, 2630–2637. [Google Scholar] [CrossRef]
- Tsounidi, D.; Koukouvinos, G.; Petrou, P.; Misiakos, K.; Zisis, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Rapid and sensitive label-free determination of aflatoxin M1 levels in milk through a White Light Reflectance Spectroscopy immunosensor. Sens. Actuators B Chem. 2019, 282, 104–111. [Google Scholar] [CrossRef]
- Ghauri, M.D.; Hussain, S.Z.; Ullah, U.; Ayaz, R.M.A.; Saleem, R.S.Z.; Kiraz, A.; Cheema, M.I. Detection of aflatoxin M1 by fiber cavity attenuated phase shift spectroscopy. Opt. Express 2021, 29, 3873–3881. [Google Scholar] [CrossRef]
- Chalyan, T.; Pasquardini, L.; Gandolfi, D.; Guider, R.; Samusenko, A.; Zanetti, M.; Pucker, G.; Pederzolli, C.; Pavesi, L. Aptamer-and Fab’-functionalized microring resonators for Aflatoxin M1 detection. IEEE J. Sel. 2017, 23, 350–357. [Google Scholar] [CrossRef]
- Misiakos, K.; Raptis, I.; Makarona, E.; Botsialas, A.; Salapatas, A.; Oikonomou, P.; Psarouli, A.; Petrou, P.S.; Kakabakos, S.E.; Tukkiniemi, K.; et al. All-silicon monolithic Mach-Zehnder interferometer as a refractive index and bio-chemical sensor. Opt. Express 2014, 22, 26803. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Botsialas, A.; Salapatas, A.; Petrou, P.S.; Haasnoot, W.; Makarona, E.; Jobst, G.; Goustouridis, D.; Siafaka-Kapadai, A.; Raptis, I.; et al. Assessment of goat milk adulteration with a label-free monolithically integrated optoelectronic biosensor. Anal. Bioanal. Chem. 2015, 407, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.S.; Raptis, I.; Misiakos, K.; Livaniou, E.; Makarona, E.; Kakabakos, S. Rapid detection of mozzarella and feta cheese adulteration with cow milk through a silicon photonic immunosensor. Analyst 2021, 146, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.S.; Makarona, E.; Haasnoot, W.; Moser, I.; Jobst, G.; Goustouridis, D.; Lees, M.; Kalatzi, K.; Raptis, I.; et al. Ultrafast multiplexed-allergen detection through advanced fluidic design and monolithic interferometric silicon chips. Anal. Chem. 2018, 90, 9559–9567. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.; Misiakos, K.; Raptis, I.; Kakabakos, S. Simultaneous Detection of Salmonella typhimurium and Escherichia coli O157:H7 in Drinking Water and Milk with Mach-Zehnder Interferometers Monolithically Integrated on Silicon Chips. Biosensors 2022, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Calderara, M.; Baggiani, C.; Giovannoli, C.; Arletti, E.; Giraudi, G. Development and application of solvent-free extraction for the detection of aflatoxin M1 in dairy products by enzyme immunoassay. J. Agric. Food Chem. 2008, 56, 1852–1857. [Google Scholar] [CrossRef]
- Wang, Y.; Dostálek, J.; Knoll, W. Long range surface plasmon-enhanced fluorescence spectroscopy for the detection of aflatoxin M1 in milk. Biosens. Bioelectron. 2009, 24, 2264–2267. [Google Scholar] [CrossRef]
- Lou, X.; Zhu, A.; Wang, H.; Wu, J.; Zhou, L.; Long, F. Direct and ultrasensitive optofluidic-based immunosensing assay of aflatoxin M1 in dairy products using organic solvent extraction. Anal. Chim. Acta 2016, 940, 120–127. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, X.; Zhang, Y.; Song, B.; Zhang, J.; Shi, H. Highly sensitive and simultaneous detection of melamine and aflatoxin M1 in milk products by multiplexed planar waveguide fluorescence immunosensor (MPWFI). Food Chem. 2016, 197, 359–366. [Google Scholar] [CrossRef] [PubMed]






| Detection Principle | Label | Sample | LOD (pg/mL) | Assay Time (min) | Ref. |
|---|---|---|---|---|---|
| Long-range surface plasmon-enhanced fluorescence spectroscopy | Cy5-GaR | Milk (defatted) | 0.6 | 53 | [41] |
| Evanescent-wave fiber-optic | Cy5.5 | Milk, Cheese, Milk tea (extraction with organic solvents and 20 times extract dilution) | 50 | 8 | [42] |
| Surface Plasmon Resonance (SPR) | Au NPs | Milk (defatted) | 18 | 55 | [28] |
| Planar waveguide fluorescence (MPWFI) | Cy5.5 | Milk (extracted and 50 times diluted milk) | 55 | 17 | [43] |
| White Light Reflectance Spectroscopy (WLRS) | - | Milk | 6 | 25 | [32] |
| Fab functionalized Si3N4 Asymmetric Mach-Zehnder Interferometer (MZI) | - | Milk (defatted, 20 times pre-concentrated/purified eluents) | 16.8 | 1.5 | [29] |
| Surface Plasmon Resonance (SPR) | - | Milk/ Milk powder (defatted, immunoaffinity separation of AFM1 prior to analysis) | 100 | 10 | [31] |
| Mach–Zehnder Interferometer (MZI) | - | Milk, chocolate milk Yogurt | 5 10 | 15 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, M.; Kourti, D.; Misiakos, K.; Economou, A.; Petrou, P.; Kakabakos, S. Mach-Zehnder Interferometric Immunosensor for Detection of Aflatoxin M1 in Milk, Chocolate Milk, and Yogurt. Biosensors 2023, 13, 592. https://doi.org/10.3390/bios13060592
Angelopoulou M, Kourti D, Misiakos K, Economou A, Petrou P, Kakabakos S. Mach-Zehnder Interferometric Immunosensor for Detection of Aflatoxin M1 in Milk, Chocolate Milk, and Yogurt. Biosensors. 2023; 13(6):592. https://doi.org/10.3390/bios13060592
Chicago/Turabian StyleAngelopoulou, Michailia, Dimitra Kourti, Konstantinos Misiakos, Anastasios Economou, Panagiota Petrou, and Sotirios Kakabakos. 2023. "Mach-Zehnder Interferometric Immunosensor for Detection of Aflatoxin M1 in Milk, Chocolate Milk, and Yogurt" Biosensors 13, no. 6: 592. https://doi.org/10.3390/bios13060592