Data Processing of SPR Curve Data to Maximize the Extraction of Changes in Electrochemical SPR Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of EC-SPR Measurement Chip
2.2. Sample Flow Handling System
2.3. EC-SPR Measurement
2.4. Data Analysis of EC-SPR Measurement
3. Results
3.1. Standard SPR Measurement at the Electron Mediator Polymer-Treated Electrode
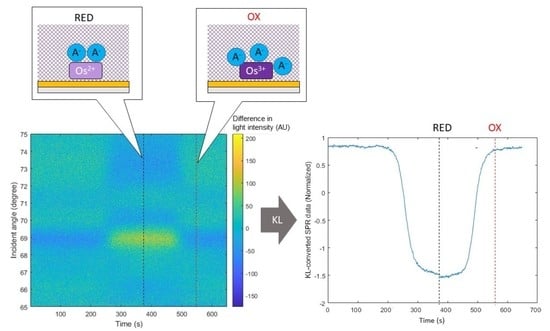
3.2. Data Processing Using KL Conversion
3.3. H2O2 Detection Using EC-SPR and KL-Converted SPR Data
3.4. Glutamate Detection by Using EC-SPR and KL-Converted SPR Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Patching, S.G. Surface plasmon resonance spectroscopy for characterisation of membrane protein–ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta 2014, 1838, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piliarik, M.; Párová, L.; Homola, J. High-throughput SPR sensor for food safety. Biosens. Bioelectron. 2009, 24, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Bellassai, N.; D’Agata, R.; Jungbluth, V.; Spoto, G. Surface Plasmon Resonance for Biomarker Detection: Advances in Non-invasive Cancer Diagnosis. Front. Chem. 2019, 7, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiuchi, T.; Tobita, T.; Miura, T.; Iwasaki, Y.; Seyama, M.; Inoue, S.; Takahashi, J.; Haga, T.; Tamechika, E. Floating Chip Mounting System Driven by Repulsive Force of Permanent Magnets for Multiple On-Site SPR Immunoassay Measurements. Sensors 2012, 12, 13964–13984. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, T.; Miura, T.; Iwasaki, Y.; Seyama, M.; Inoue, S.; Takahashi, J.; Haga, T.; Tamechika, E. Passive Fluidic Chip Composed of Integrated Vertical Capillary Tubes Developed for On-Site SPR Immunoassay Analysis Targeting Real Samples. Sensors 2012, 12, 7095–7108. [Google Scholar] [CrossRef]
- Miura, T.; Horiuchi, T.; Iwasaki, Y.; Seyama, M.; Camou, S.; Takahashi, J.; Haga, T. Patterned cellulose membrane for surface plasmon resonance measurement. Sens. Actuators B 2012, 173, 354–360. [Google Scholar] [CrossRef]
- Inoue, S.; Seyama, M.; Miura, T.; Horiuchi, T.; Iwasaki, Y.; Takahashi, J.; Hayashi, K.; Tamechika, E. A reliable aptamer array prepared by repeating inkjet-spotting toward on-site measurement. Biosens. Bioelectron. 2016, 85, 943–949. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Seyama, M.; Matsuura, N.; Inoue, S.; Hayashi, K.; Koizumi, H. Direct Measurement of Near-Wall Molecular Transport Rate in a Microchannel and Its Dependence on Diffusivity. Langmuir 2021, 37, 8687–8695. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Horiuchi, T.; Niwa, O. Detection of electrochemical enzymatic reactions by surface plasmon resonance measurement. Anal. Chem. 2001, 73, 1595–1598. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, G.; Feng, X.; Du, W.; Liu, X.; Liu, B.-F. Recent advances in microchip electrophoresis for amino acid analysis. Anal. Bioanal. Chem. 2013, 405, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, S.; Suzuki, M.; Asano, Y. Application of an enzyme chip to the microquantification of L-phenylalanine. Anal. Biochem. 2006, 359, 72–78. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Tretjakov, A.; Syritski, V. Enhancing binding properties of imprinted polymers for the detection of small molecules. Proc. Est. Acad. Sci. 2018, 1, 138–146. [Google Scholar] [CrossRef]
- Iha, K.; Inada, M.; Kawada, N.; Nakaishi, K.; Watabe, S.; Tan, Y.H.; Shen, C.; Ke, L.Y.; Yoshimura, T.; Ito, E. Ultrasensitive ELISA Developed for Diagnosis. Diagnostics 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Munir, A.; Li, Z.; Zhou, H.S. Aptamer–Au NPs conjugates-enhanced SPR sensing for the ultrasensitive sandwich immunoassay. Biosens. Bioelectron. 2009, 25, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Feng, F.; Zhao, L.; Wang, C.; Wang, H.; Tian, M.; Qin, J.; Duan, Y.; He, X. Aptamer/thrombin/aptamer-AuNPs sandwich enhanced surface plasmon resonance sensor for the detection of subnanomolar thrombin. Biosens. Bioelectron. 2013, 47, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chieng, A.; Chiang, M.; Triloges, K.; Chang, M.; Wang, Y. Recent progress in the studies of electrochemical interfaces by surface plasmon resonance spectroscopy and microscopy. Curr. Opin. Electrochem. 2019, 13, 94–99. [Google Scholar] [CrossRef]
- Juan-Colás, J.; Johnson, S.; Krauss, T.F. Dual-Mode Electro-Optical Techniques for Biosensing Applications: A Review. Sensors 2017, 17, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastpar, M. The Distributed Karhunen–Loève Transform. IEEE Trans. Inf. Theory 2006, 52, 5177–5196. [Google Scholar] [CrossRef] [Green Version]
- Nunez-Carmona, E.; Abbatabgelo, M.; Sberveglieri, V. Innovative Sensor Approach to Follow Campylobacter jejuni Developlemt. Biosensors 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martynko, E.; Kirsanov, D. Application of Chemometrics in Biosensing: A Brief Review. Biosensors 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Corradi, E.; Agostini, M.; Greco, G.; Massidda, D.; Santi, M.; Calderisi, M.; Signore, G.; Cecchini, M. An objective, principal-component-analysis (PCA) based, method which improves the quartz-crystal-microbalance (QCM) sensing performance. Sens. Actuators A 2020, 315, 112323. [Google Scholar] [CrossRef]
- Rosandi, V.A.; Linda, T.M.; Agustirandi, B.; Umar, L. Simple Amperometric Biosensor for Sucrose Concentration Measurement Based on Principal Component Analysis. J. Phys. Conf. Ser. 2021, 2049, 012048. [Google Scholar] [CrossRef]
- Zaharov, V.V.; Farahi, R.H.; Snyder, P.; Davison, B.H.; Passian, A. Karhunen-Loeve treatment to remove noise and facilitate data analysis in sensing, spectroscopy and other applications. Analyst 2014, 139, 22. [Google Scholar] [CrossRef]
- Kurihara, K.; Suzuki, K. Theoretical understanding of an absorption-based surface plasmon resonance sensor based on Kretchmann’s theory. Anal. Chem. 2002, 74, 696–701. [Google Scholar] [CrossRef]
- Wang, R. Introduction to Orthogonal Transforms: With Applications in Data Processing and Analysis; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Wurtman, R.J.; Rose, C.M.; Chou, C.; Larin, F.F. Daily rhythms in the concentrations of various amino acids in human plasma. N. Engl. J. Med. 1968, 279, 171–175. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, S.; Fukada, K.; Hayashi, K.; Seyama, M. Data Processing of SPR Curve Data to Maximize the Extraction of Changes in Electrochemical SPR Measurements. Biosensors 2022, 12, 615. https://doi.org/10.3390/bios12080615
Inoue S, Fukada K, Hayashi K, Seyama M. Data Processing of SPR Curve Data to Maximize the Extraction of Changes in Electrochemical SPR Measurements. Biosensors. 2022; 12(8):615. https://doi.org/10.3390/bios12080615
Chicago/Turabian StyleInoue, Suzuyo, Kenta Fukada, Katsuyoshi Hayashi, and Michiko Seyama. 2022. "Data Processing of SPR Curve Data to Maximize the Extraction of Changes in Electrochemical SPR Measurements" Biosensors 12, no. 8: 615. https://doi.org/10.3390/bios12080615