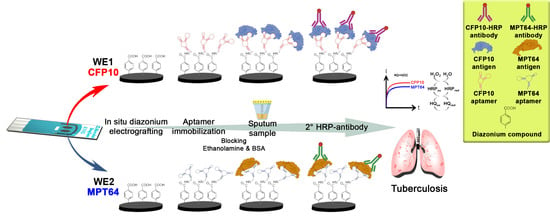
Simultaneous Amperometric Aptasensor Based on Diazonium Grafted Screen-Printed Carbon Electrode for Detection of CFP10 and MPT64 Biomarkers for Early Tuberculosis Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Preparation of Dual Aptasensor Based on the Carboxyphenyl-Modified Carbon Electrode
2.4. Characterization of Dual-Carboxyphenyl Diazonium Fabricated Electrode
2.5. Simultaneous Detection of CFP10 and MPT64 Antigens
2.6. Clinical Samples
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Diazonium-Modified Dual Aptasensor
3.2. Electrochemical Characterization of Diazonium-Modified Dual Aptasensor
3.3. Optimization of the Aptasensor Variables

3.4. Analytical Performance of the CFP10 and MPT64 Dual Aptasensor
3.5. Selectivity and Reproducibility Study of the Aptasensor
3.6. Simultenoues Detection of CFP10 and MPT64 in Clinical Sputum Samples

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; CIP Data. 2021. Available online: http://apps.who.int/iris (accessed on 8 September 2022).
- Prussin, A.J.; Marr, L.C. Sources of Airborne Microorganisms in the Built Environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, S.; Tanveer Hossain Parash, M.; Emran, N.A.; Tofazzal Hossain, A.B.M.; Shimmi, S.C. Diagnostic Challenges and Gene-Xpert Utility in Detecting Mycobacterium Tuberculosis among Suspected Cases of Pulmonary Tuberculosis. PLoS ONE 2021, 16, e0251858. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.M.; Weigel, K.M.; Lakey Becker, A.; Ontengco, D.; Narita, M.; Tolstorukov, I.; Doebler, R.; Cangelosi, G.A.; Niemz, A. Pilot Study of a Rapid and Minimally Instrumented Sputum Sample Preparation Method for Molecular Diagnosis of Tuberculosis. Sci. Rep. 2016, 6, 19541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urdea, M.; Penny, L.A.; Olmsted, S.S.; Giovanni, M.Y.; Kaspar, P.; Shepherd, A.; Wilson, P.; Dahl, C.A.; Buchsbaum, S.; Moeller, G.; et al. Requirements for High Impact Diagnostics in the Developing World. Nature 2006, 444, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Schiller, I.; Horne, D.J.; Pai, M.; Boehme, C.C.; Dendukuri, N. Xpert® MTB/RIF Assay for Pulmonary Tuberculosis and Rifampicin Resistance in Adults. Cochrane Database Syst. Rev. 2014, 2014, CD009593. [Google Scholar] [CrossRef]
- Zhou, W.; Jimmy Huang, P.J.; Ding, J.; Liu, J. Aptamer-Based Biosensors for Biomedical Diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [Green Version]
- Yunus, M.H.; Yusof, N.A.; Raston, N.H.A.; Noor, S.S.M.; Sulaiman, Y.; Abdullah, J. A Novel Amperometric Aptamer-Antibody Sandwich Assay for the Detection of Tuberculosis with Diazonium Electrografted Enhanced Modified Electrode. IEEE Sens. J. 2021, 21, 22442–22449. [Google Scholar] [CrossRef]
- Kumari, P.; Lavania, S.; Tyagi, S.; Dhiman, A.; Rath, D.; Anthwal, D.; Gupta, R.K.; Sharma, N.; Gadpayle, A.K.; Taneja, R.S.; et al. A Novel Aptamer-Based Test for the Rapid and Accurate Diagnosis of Pleural Tuberculosis. Anal. Biochem. 2019, 564–565, 80–87. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Duan, S.; Su, L.; Zhang, J.; He, F. Mycobacterium Tuberculosis Strain H37Rv Electrochemical Sensor Mediated by Aptamer and AuNPs–DNA. ACS Sens. 2019, 4, 849–855. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.; Sun, D.; Yu, W.; Tan, W.; Luo, Z.; Chen, Z. Dual-Aptamer-Based Voltammetric Biosensor for the Mycobacterium Tuberculosis Antigen MPT64 by Using a Gold Electrode Modified with a Peroxidase Loaded Composite Consisting of Gold Nanoparticles and a Zr(IV)/Terephthalate Metal-Organic Framework. Microchim. Acta 2018, 185, 543. [Google Scholar] [CrossRef]
- Ansari, N.; Ghazvini, K.; Ramezani, M.; Shahdordizadeh, M.; Yazdian-Robati, R.; Abnous, K.; Taghdisi, S.M. Selection of DNA Aptamers against Mycobacterium Tuberculosis Ag85A, and Its Application in a Graphene Oxide-Based Fluorometric Assay. Microchim. Acta 2017, 185, 21. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, S.; Lee, C.H.; Chuang, T.L.; Hsueh, P.R.; Lai, H.C.; Lin, C.W. Amplified Surface Plasmon Resonance Immunosensor for Interferon-Gamma Based on a Streptavidin-Incorporated Aptamer. Biosens. Bioelectron. 2012, 37, 68–74. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.H. Liquid Crystal-Based Aptasensor for the Detection of Interferon-γ and Its Application in the Diagnosis of Tuberculosis Using Human Blood. Sens. Actuators B Chem. 2019, 282, 574–579. [Google Scholar] [CrossRef]
- Li, J.; Hu, K.; Zhang, Z.; Teng, X.; Zhang, X. Click DNA Cycling in Combination with Gold Nanoparticles Loaded with Quadruplex DNA Motifs Enable Sensitive Electrochemical Quantitation of the Tuberculosis-Associated Biomarker CFP-10 in Sputum. Microchim. Acta 2019, 186, 662. [Google Scholar] [CrossRef]
- Thakur, H.; Kaur, N.; Sabherwal, P.; Sareen, D.; Prabhakar, N. Aptamer Based Voltammetric Biosensor for the Detection of Mycobacterium Tuberculosis Antigen MPT64. Microchim. Acta 2017, 184, 1915–1922. [Google Scholar] [CrossRef]
- Sypabekova, M.; Jolly, P.; Estrela, P.; Kanayeva, D. Electrochemical Aptasensor Using Optimized Surface Chemistry for the Detection of Mycobacterium Tuberculosis Secreted Protein MPT64 in Human Serum. Biosens. Bioelectron. 2019, 123, 141–151. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Guo, S.; Cao, J.; Zhou, J.; Zuo, J.; Bai, L. A Sandwich-Type Electrochemical Aptasensor for Mycobacterium Tuberculosis MPT64 Antigen Detection Using C60NPs Decorated N-CNTs/GO Nanocomposite Coupled with Conductive PEI-Functionalized Metal-Organic Framework. Biomaterials 2019, 216, 119253. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Feng, Y.; Yao, Q.Q.; He, F. Selection of a New Mycobacterium Tuberculosis H37Rv Aptamer and Its Application in the Construction of a SWCNT/Aptamer/Au-IDE MSPQC H37Rv Sensor. Biosens. Bioelectron. 2017, 98, 261–266. [Google Scholar] [CrossRef]
- Sypabekova, M.; Dukenbayev, K.; Tsepke, A.; Akisheva, A.; Oralbayev, N.; Kanayeva, D. An Aptasensor for the Detection of Mycobacterium Tuberculosis Secreted Immunogenic Protein MPT64 in Clinical Samples towards Tuberculosis Detection. Sci. Rep. 2019, 9, 16273. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Liu, J.; Ling, Y.; Yang, H.; Liu, Z.; Zheng, R.; Qin, L.; Hu, Z. Evaluation of the Clinical Value of ELISA Based on MPT64 Antibody Aptamer for Serological Diagnosis of Pulmonary Tuberculosis. BMC Infect. Dis. 2012, 12, 96. [Google Scholar] [CrossRef]
- Li, L.; Yuan, Y.; Chen, Y.; Zhang, P.; Bai, Y.; Bai, L. Aptamer Based Voltammetric Biosensor for Mycobacterium Tuberculosis Antigen ESAT-6 Using a Nanohybrid Material Composed of Reduced Graphene Oxide and a Metal-Organic Framework. Microchim. Acta 2018, 185, 379. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Afzal, M.; Khalid, R.; Akhtar, M.W.; Qazi, M.H. Potential of Multi-Component Antigens for Tuberculosis Diagnosis. Biologicals 2017, 48, 109–113. [Google Scholar] [CrossRef]
- Oliveira Tavares, R.C.; Salgado, J.; Moreira, V.B.; Ferreira, M.A.S.; Queiroz Mello, F.C.; Leung, J.W.; Fonseca, L.D.S.; Spallek, R.; Singh, M.; Saad, M.H.F. Interferon Gamma Response to Combinations 38 KDa/CFP-10, 38 KDa/MPT-64, ESAT-6/MPT-64 and ESAT-6/CFP-10, Each Related to a Single Recombinant Protein of Mycobacterium Tuberculosis in Individuals from Tuberculosis Endemic Areas. Microbiol. Immunol. 2007, 51, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.I.F.; Melo, E.S.P.; Ramos, C.A.N.; Farias, T.A.; Osório, A.L.A.R.; Jorge, K.S.G.; Vidal, C.E.S.; Silva, A.S.; Silva, M.R.; Pellegrin, A.O.; et al. Screening of Recombinant Proteins as Antigens in Indirect ELISA for Diagnosis of Bovine Tuberculosis. Springerplus 2012, 1, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunus, M.H.; Tan Farrizam, S.N.; Abdul Karim, I.Z.; Noordin, R. A Lateral Flow Rapid Test for Human Toxocariasis Developed Using Three Toxocara Canis Recombinant Antigens. Am. J. Trop. Med. Hyg. 2018, 98, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Yunus, M.H.; Yusof, N.A.; Ismail, S.; Suraiya, S.; Noor, M.; Mohammad, F.; Sulaiman, Y.; Hanun, N.; Raston, A.; Abdullah, J.; et al. Surface-Enhanced Carboxyphenyl Diazonium Functionalized Screen-Printed Carbon Electrode for the Screening of Tuberculosis in Sputum Samples. Nanomaterials 2022, 12, 2551. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, J.; Liu, M.; Wu, Y.; Shen, Z.; Li, G. Sensitive Detection of Human Breast Cancer Cells Based on Aptamer–Cell–Aptamer Sandwich Architecture. Anal. Chim. Acta 2013, 764, 59–63. [Google Scholar] [CrossRef]
- Laurenti, E.; Ghibaudi, E.; Todaro, G.; Pia Ferrari, R. Enzymatic Degradation of 2,6-Dichlorophenol by Horseradish Peroxidase: UV–Visible and Mass Spectrophotometric Characterization of the Reaction Products. J. Inorg. Biochem. 2002, 92, 75–81. [Google Scholar] [CrossRef]
- Ferrari, R.P.; Laurenti, E.; Trotta, F.; Ferrari, R.P.; Laurenti, E.; Trotta, F. Oxidative 4-Dechlorination of 2,4,6-Trichlorophenol Catalyzed by Horseradish Peroxidase. JBIC J. Biol. Inorg. Chem. 1999, 4, 232–237. [Google Scholar] [CrossRef]
- Wei, X.; Liu, T.; Li, J.; Chen, X. A Magnetic-Controlled Amperometric Biosensor Based on Composite Bio-Particulates Fe3O4 and Nano-Au with the Signal Enhancement by Increasing Loading of Horseradish Peroxidase. Int. J. Electrochem. Sci. 2011, 6, 4953–4966. [Google Scholar]
- Lei, C.-X.; Hu, S.-Q.; Gao, N.; Shen, G.-L.; Yu, R.-Q. An Amperometric Hydrogen Peroxide Biosensor Based on Immobilizing Horseradish Peroxidase to a Nano-Au Monolayer Supported by Sol–Gel Derived Carbon Ceramic Electrode. Bioelectrochemistry 2004, 65, 33–39. [Google Scholar] [CrossRef]
- Delamar, M.; Hitmi, R.; Pinson, J.; Saveant, J.M. Covalent Modification of Carbon Surfaces by Grafting of Functionalized Aryl Radicals Produced from Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1992, 114, 5883–5884. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A Powerful Method for Surface Modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef]
- Galdino, F.E.; Smith, J.P.; Kwamou, S.I.; Kampouris, D.K.; Iniesta, J.; Smith, G.C.; Bonacin, J.A.; Banks, C.E. Graphite Screen-Printed Electrodes Applied for the Accurate and Reagentless Sensing of PH. Anal. Chem. 2015, 87, 11666–11672. [Google Scholar] [CrossRef] [Green Version]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Electrochemical Performance of Activated Screen Printed Carbon Electrodes for Hydrogen Peroxide and Phenol Derivatives Sensing. J. Electroanal. Chem. 2019, 839, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Gillan, L.; Teerinen, T.; Johansson, L.-S.; Smolander, M. Controlled Diazonium Electrodeposition towards a Biosensor for C-Reactive Protein. Sens. Int. 2021, 2, 100060. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, C.; Yang, H.; Hu, H.; Feng, Y.; Qin, L.; Cui, Z.; Bi, A.; Zheng, R.; Jin, R.; et al. Clinical Value of ELISA-MPT64 for the Diagnosis of Tuberculous Pleurisy. Curr. Microbiol. 2012, 65, 313–318. [Google Scholar] [CrossRef]
- Mohd Azmi, U.Z.; Yusof, N.A.; Kusnin, N.; Abdullah, J.; Suraiya, S.; Ong, P.S.; Ahmad Raston, N.H.; Abd Rahman, S.F.; Mohamad Fathil, M.F. Sandwich Electrochemical Immunosensor for Early Detection of Tuberculosis Based on Graphene/Polyaniline-Modified Screen-Printed Gold Electrode. Sensors 2018, 18, 3926. [Google Scholar] [CrossRef] [Green Version]
- Azmi, U.Z.M.; Yusof, N.A.; Abdullah, J.; Mohammad, F.; Ahmad, S.A.A.; Suraiya, S.; Raston, N.H.A.; Faudzi, F.N.M.; Khiste, S.K.; Al-Lohedan, H.A. Aptasensor for the Detection of Mycobacterium Tuberculosis in Sputum Utilising Cfp10-Esat6 Protein as a Selective Biomarker. Nanomaterials 2021, 11, 2446. [Google Scholar] [CrossRef]
- Chutichetpong, P.; Cheeveewattanagul, N.; Srilohasin, P.; Rijiravanich, P.; Chaiprasert, A.; Surareungchai, W. Rapid Screening Drug Susceptibility Test in Tuberculosis Using Sandwich Electrochemical Immunosensor. Anal. Chim. Acta 2018, 1025, 108–117. [Google Scholar] [CrossRef]
- Mohamad, S.; Azmi, N.C.; Noordin, R. Development and Evaluation of a Sensitive and Specific Assay for Diagnosis of Human Toxocariasis by Use of Three Recombinant Antigens (TES-26, TES-30USM, and TES-120). J. Clin. Microbiol. 2009, 47, 1712. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Ranganathan, U.D.; Bethunaickan, R. Improved Diagnosis of Pulmonary Tuberculosis by Detection of Antibodies against Multiple Mycobacterium Tuberculosis Antigens. Diagn. Microbiol. Infect. Dis. 2008, 60, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azmi, U.Z.; Yusof, N.A.; Abdullah, J.; Alang Ahmad, S.A.; Mohd Faudzi, F.N.; Ahmad Raston, N.H.; Suraiya, S.; Ong, P.S.; Krishnan, D.; Sahar, N.K. Portable Electrochemical Immunosensor for Detection of Mycobacterium Tuberculosis Secreted Protein CFP10-ESAT6 in Clinical Sputum Samples. Microchim. Acta 2021, 188, 20. [Google Scholar] [CrossRef] [PubMed]
- Lavania, S.; Das, R.; Dhiman, A.; Myneedu, V.P.; Verma, A.; Singh, N.; Sharma, T.K.; Tyagi, J.S. Aptamer-Based TB Antigen Tests for the Rapid Diagnosis of Pulmonary Tuberculosis: Potential Utility in Screening for Tuberculosis. ACS Infect. Dis. 2018, 4, 1718–1726. [Google Scholar] [CrossRef]
- Torati, S.R.; Reddy, V.; Yoon, S.S.; Kim, C. Electrochemical Biosensor for Mycobacterium Tuberculosis DNA Detection Based on Gold Nanotubes Array Electrode Platform. Biosens. Bioelectron. 2016, 78, 483–488. [Google Scholar] [CrossRef]








| Detection Technique | Target | Linear Range | LOD | Diagnostic Performance | Reference | |
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | |||||
| DPV | CFP10-ESAT6 | 5–500 ng ml−1 | 1.5 ng mL−1 | 100% (n = 6) | 100% (n = 4) | [40] |
| DPV | CFP10-ESAT6 | 10–500 ng mL−1 | 1.5 ng mL−1 | 100% (n = 6) | 91.7% (n = 11) | [44] |
| EIS | MPT64 | 0.1 fM–1 nM | 4.1 fM | Sputum: 76.47% Serum: 88.24% (n = 17) | Sputum: 100% Serum: 100% (n = 4) | [20] |
| DPV | HspX | 13 pM–648 nM | 13 pM | 92.3% (n = 13) | 91.2% (n = 57) | [45] |
| DPV | M. tuberculosis DNA | 0.01–100 ng µL−1 | 0.05 ng μL−1 | – | – | [46] |
| DPV | CFP10 | 20–100 ng mL−1 | 15 ng mL−1 | – | – | [39] |
| CA | CFP10 and MPT64 | CFP10: 0.5–100 ng mL−1 MPT64: 0.75–250 ng mL−1 | CFP10: 1.68 ng mL−1 MPT64: 1.82 ng mL−1 | CFP10 WE: 95.8% MPT64 WE: 91.7% Overall, CFP10+MPT64: 100% (n = 24) | CFP10 WE: 100% MPT64 WE: 100% Overall, CFP10+MPT64: 100% (n = 13) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunus, M.H.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ahmad Raston, N.H.; Md Noor, S.S. Simultaneous Amperometric Aptasensor Based on Diazonium Grafted Screen-Printed Carbon Electrode for Detection of CFP10 and MPT64 Biomarkers for Early Tuberculosis Diagnosis. Biosensors 2022, 12, 996. https://doi.org/10.3390/bios12110996
Yunus MH, Yusof NA, Abdullah J, Sulaiman Y, Ahmad Raston NH, Md Noor SS. Simultaneous Amperometric Aptasensor Based on Diazonium Grafted Screen-Printed Carbon Electrode for Detection of CFP10 and MPT64 Biomarkers for Early Tuberculosis Diagnosis. Biosensors. 2022; 12(11):996. https://doi.org/10.3390/bios12110996
Chicago/Turabian StyleYunus, Muhammad Hafiznur, Nor Azah Yusof, Jaafar Abdullah, Yusran Sulaiman, Nurul Hanun Ahmad Raston, and Siti Suraiya Md Noor. 2022. "Simultaneous Amperometric Aptasensor Based on Diazonium Grafted Screen-Printed Carbon Electrode for Detection of CFP10 and MPT64 Biomarkers for Early Tuberculosis Diagnosis" Biosensors 12, no. 11: 996. https://doi.org/10.3390/bios12110996