Mechanical Force-Induced Blue-Shifted and Enhanced Emission for AIEgens
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Photophysical Spectra
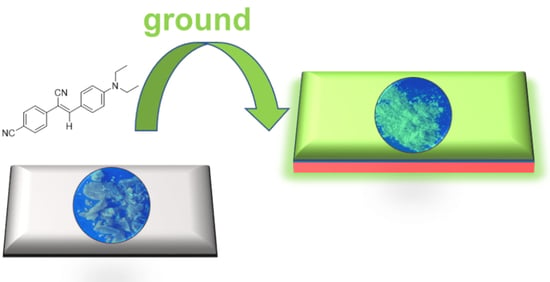
3.2. MC Behaviors of Molecules 1–6 under Grinding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.-G.; Zhang, X.-Q.; Xu, B.-J.; Zhou, X.; Ma, C.-P.; Zhang, Y.; Liu, S.-W.; Xu, J.-R. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef]
- Ooyamaa, Y.; Harima, Y. Molecular design of mechanofluorochromic dyes and their solid-state fluorescence properties. J. Mater. Chem. 2011, 21, 8372–8380. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Wang, Z.-J.; Teng, M.-J.; Xu, Z.-J.; Jia, X.-R. Mechanically Induced Multicolor Change of Luminescent Materials. ChemPhysChem 2015, 16, 1811–1828. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular Conformation and Packing: Their Critical Roles in the Emission Performance of Mechanochromic Fluorescence Materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, Z.-G.; Zhang, Y.; Liu, S.; Xu, J. Recent Advances in Mechanochromic Luminescent Metal Complexes. J. Mater. Chem. C 2013, 1, 3376–3390. [Google Scholar] [CrossRef]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-Containing Polymers: Methods for Preparation of Mechanochromic Materials. Chem. Soc. Rev. 2013, 42, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Wang, Z.-J.; Meng, X.; Ma, Z.-M.; Xu, Z.-J.; Ma, Y.-G.; Jia, X.-Y. A Mechanochromic Single Crystal: Turning Two Color Changes into a Tricolored Switch. Angew. Chem. Int. Ed. 2016, 55, 519–522. [Google Scholar] [CrossRef]
- Ito, H.; Muromoto, M.; Kurenuma, S.; Ishizaka, S.; Kitamura, N.; Sato, H.; Seki, T. Mechanical Stimulation and Solid Seeding Trigger Single-crystal-to-single-crystal Molecular Domino Transformations. Nat. Commun. 2013, 4, 2009. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; He, B.-Z.; Wu, W.-J.; Alam, P.; Zhang, H.; Gong, J.-Y.; Song, F.-Y.; Wang, Z.-Y.; Sung, H.H.Y.; Williams, I.D.; et al. Molecular Motions in AIEgen Crystals: Turning on Photoluminescence by Force-Induced Filament Sliding. J. Am. Chem. Soc. 2020, 142, 14608–14618. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, C.; Fu, Z.; Zhang, H.; Wang, J.; Feng, H.-T.; Wang, K.; Zou, B.; Lam, J.W.Y.; Tang, B.Z. A Synergy Between the Push-Pull Electronic Effect and Twisted Conformation for High-Contrast Mechanochromic AIEgens. Mater. Horiz. 2021, 8, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-J.; Xu, B.; Zhang, J.-B.; Tan, X.; Wang, L.-J.; Chen, J.-L.; Lv, H.G.; Wen, S.-P.; Li, B.; Ye, L.; et al. Piezochromic luminescence based on the molecular aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angew. Chem. Int. Ed. 2012, 51, 10782. [Google Scholar] [CrossRef] [PubMed]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct responses to mechanical grinding and hydrostatic pressure in luminescent chromism of tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322–10325. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, K.; Yang, K.; Liu, B.; Zou, B. Luminescence properties of compressed tetraphenylethene: The role of intermo- lecular interactions. J. Phys. Chem. Lett. 2014, 5, 2968–2973. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, Y.; Luo, S.; Gao, Y.; Gao, N.; Wang, K.; Zou, B.; Yang, B.; Ma, Y. Rehybridization of nitrogen atom induced photoluminescence enhancement under pressure stimulation. Adv. Funct. Mater. 2017, 27, 1602276. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, K.; Dai, Y.; Xiao, G.; Ma, Y.; Qiao, Y.; Zou, B. Pressure-induced emission enhancement of carbazole: The restriction of intramolecular vibration. J. Phys. Chem. Lett. 2017, 8, 4191–4196. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, H.; Qiu, R.; Liu, Z.; Wang, C.; Katsura, T.; Zhang, H.; Wu, M.; Yao, M.; Zheng, H.; et al. Pressure-induced emission enhancement and multicolor emission for 1,2,3,4-tetraphenyl-1,3-cyclopentadiene: Con- trolled structure evolution. J. Phys. Chem. Lett. 2019, 10, 5557–5562. [Google Scholar] [CrossRef]
- Lu, S.; Xiao, G.; Sui, L.; Feng, T.; Yong, X.; Zhu, S.; Li, B.; Liu, Z.; Zou, B.; Jin, M.; et al. Piezochromic Carbon Dots with Two-photon Fluorescence. Angew. Chem. Int. Ed. 2017, 56, 6187–6191. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, G.-J.; Yang, M.; Zou, B.; Zhang, Z.-L.; Pang, D.W. Mechanofluorochromic Carbon Nanodots: Controllable Pressure-Triggered Blue- and Red-Shifted Photoluminescence. Angew. Chem. Int. Ed. 2018, 57, 1893–1897. [Google Scholar] [CrossRef]
- Jing, P.; Han, D.; Li, D.; Zhou, D.; Shen, D.; Xiao, G.; Zou, B.; Qu, S. Surface Related Intrinsic Luminescence from Carbon Nanodots: Solvent Dependent Piezochromism. Nanoscale Horiz. 2019, 4, 175–181. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Wang, Y.; Xu, Z.; Luo, Y.; Wei, Y.; Jia, X. A Novel Mechanochromic and Photochromic Polymer Film: When Rhodamine Joins Polyurethane. Adv. Mater. 2015, 27, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, Y.; Dai, Y.; Wang, K.; Zhang, S.; Chen, G.; Zou, B.; Yang, B. Pressure-Induced Blue-Shifted and Enhanced Emission: A Cooperative Effect Between Aggregation-Induced Emission and Energy-Transfer Suppression. J. Am. Chem. Soc. 2020, 142, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, Q.; Zou, B.; Liu, Y.; Xu, B.; Tian, W. Piezochromic Luminescence of Donor-Acceptor Cocrystals: Distinct Responses to Anisotropic Grinding and Isotropic Compression. Angew. Chem. Int. Ed. 2018, 57, 15670–15674. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Zou, B.; Ye, K.-Q.; Zhang, H.-Y.; Wang, Y. Luminescent Chromism of Boron Diketonate Crystals: Distinct Responses to Different Stresses. Adv. Mater. 2015, 27, 2918–2922. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, U.; Heun, S.; Mahrt, R.F.; Scherf, U.; Hopmeier, M.; Siegner, U.; Go, E.O.; Mu, K.; Ba, H. Aggregate fluorescence in conjugated polymers. Chem. Phys. Lett. 1995, 240, 373–378. [Google Scholar] [CrossRef]
- Jakubiak, R.; Collison, C.; Wan, W.C.; Rothberg, L.J.; Hsieh, B.R. Aggregation quenching of luminescence in electroluminescent conjugated polymers. J. Phys. Chem. A 1999, 103, 2394–2398. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Q.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-penta-phenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Feng, H.-T.; Yuan, Y.-X.; Xiong, J.-B.; Zheng, Y.-S.; Tang, B.Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 2018, 47, 7452–7476. [Google Scholar] [CrossRef]
- Qi, C.; Wang, X.; Chen, Z.; Xiang, S.; Wang, T.; Feng, H.-T.; Tang, B.Z. Organometallic AIEgens for biological theranostics. Mater. Chem. Front. 2021, 5, 3281–3297. [Google Scholar] [CrossRef]
- Shen, J.; Tao, K.; Gu, P.; Gui, C.; Wang, D.; Tan, Z.; Wang, L.; Wang, z.; Qin, A.; Tang, B.Z.; et al. Aggregation- induced emission luminogen for specific identification of malignant tumour in vivo. Sci. China Chem. 2020, 63, 393–397. [Google Scholar] [CrossRef]
- Feng, H.-T.; Li, Y.; Duan, X.; Wang, X.; Qi, C.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Substitution Activated Precise Phototheranostics through Supramolecular Assembly of AIEgen and Calixarene. J. Am. Chem. Soc. 2020, 142, 15966–15974. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregatesfrom aggregation-induced emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Sun, Y.; Stang, P.J. Metallacycles, metallacages, and their aggregate/optical behaviour. Aggregate 2021, 2, e94. [Google Scholar]
- Wang, X.; Xiang, S.; Qi, C.; Chen, M.; Su, X.; Yang, J.; Tian, J.; Feng, H.-T.; Tang, B.Z. Visualization of Enantiorecognition and Resolution by Chiral AIEgens. ACS Nano 2022, 16, 8223–8232. [Google Scholar] [CrossRef]
- Yang, Z.; Chi, Z.; Mao, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Aldred, M.P.; Chi, Z. Recent advances in mechano-responsive luminescence of tetraphenylethylene derivatives with aggregation-induced emission properties. Mater. Chem. Front. 2018, 2, 861–890. [Google Scholar] [CrossRef]
- Lowe, C.; Weder, C. Oligo(p-phenylene vinylene) Excimers as Molecular Probes: Deformation-induced Color Changes in Photo- luminescent Polymer Blends. Adv. Mater. 2002, 14, 1625–1629. [Google Scholar] [CrossRef]
- Wang, C.; Xu, B.; Li, M.; Chi, Z.; Xie, Y.; Li, Q.; Li, Z. A stable tetraphenylethene derivative: Aggregation-induced emission, different crystalline polymorphs, and totally different mechanoluminescence properties. Mater. Horiz. 2016, 3, 220–225. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Chung, J.W.; Gierschner, J.; Kim, K.S.; Choi, M.-G.; Kim, D.; Park, S.Y. Multistimuli Two-Color Luminescence Switching via Different Slip-Stacking of Highly Fluorescent Molecular Sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.-S.; Su, X.-L.; Yin, Y.-T.; Zhang, B.-X.; Liu, X.-Y.; Wang, R.-P.; Chen, P.; Feng, H.-T.; Tang, B.-Z. Mechanical Force-Induced Blue-Shifted and Enhanced Emission for AIEgens. Biosensors 2022, 12, 1055. https://doi.org/10.3390/bios12111055
Guo C-S, Su X-L, Yin Y-T, Zhang B-X, Liu X-Y, Wang R-P, Chen P, Feng H-T, Tang B-Z. Mechanical Force-Induced Blue-Shifted and Enhanced Emission for AIEgens. Biosensors. 2022; 12(11):1055. https://doi.org/10.3390/bios12111055
Chicago/Turabian StyleGuo, Chang-Sheng, Xiao-Long Su, Yu-Ting Yin, Bo-Xuan Zhang, Xin-Yi Liu, Rui-Peng Wang, Pu Chen, Hai-Tao Feng, and Ben-Zhong Tang. 2022. "Mechanical Force-Induced Blue-Shifted and Enhanced Emission for AIEgens" Biosensors 12, no. 11: 1055. https://doi.org/10.3390/bios12111055