A Core–Shell Au@TiO2 and Multi-Walled Carbon Nanotube-Based Sensor for the Electroanalytical Determination of H2O2 in Human Blood Serum and Saliva
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Measurements
2.3. Synthesis of Gold Nanoparticles (AuNPs)
2.4. Synthesis of Core–Shell AuNPs@TiO2 and AuNPs@TiO2/MWCNTs
2.5. Fabrication of H2O2 Electrode (Au@TiO2/MWCNTs/GCE)
2.6. Real Sample Analysis
3. Results and Discussion
3.1. UV-Visible Spectroscopy
3.2. Raman Spectroscopy
3.3. X-ray Diffraction (XRD)
3.4. Zeta-Potential Analyzer and Size Distribution
3.5. Transmission Electron Microscopy (TEM)
3.6. Scanning Electron Microscopy and Energy Dispersive X-ray Analysis (SEM and EDX)
3.7. Atomic Force Microscopy (AFM)
3.8. Cyclic Voltammetry
3.9. Electrochemical Impedance Spectroscopy
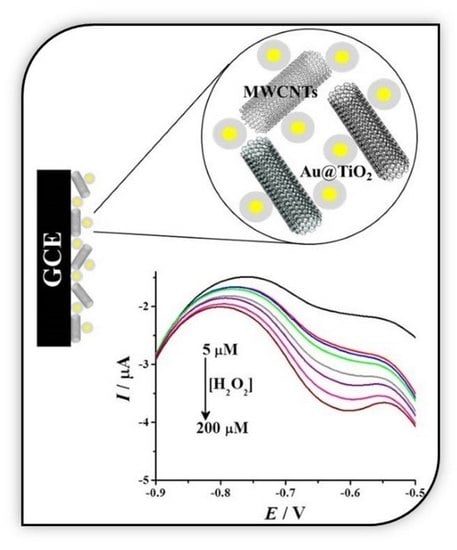
3.10. Analytical Performance (H2O2 Sensing)
3.10.1. Stability and Reproducibility of H2O2 Sensor
3.10.2. Selectivity of H2O2 Sensor
3.11. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Teradal, N.L.; Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Porto, L.S.; Silva, D.N.; de Oliveira, A.E.F.; Pereira, A.C.; Borges, K.B. Carbon nanomaterials: Synthesis and applications to development of electrochemical sensors in determination of drugs and compounds of clinical interest. Rev. Anal. Chem. 2019, 38, 20190017. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Knezevic, N.Z. Graphene-based biosensors for on-site detection of contaminants in food. Anal. Methods 2018, 10, 5061–5070. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous nanomaterials employed in the development of electrochemical sensors based on screen-printing technique—A review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Kirchner, E.-M.; Hirsch, T. Recent developments in carbon-based two-dimensional materials: Synthesis and modification aspects for electrochemical sensors. Microchim. Acta 2020, 187, 441. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Recent advances in carbon nanomaterials as electrochemical biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Pandey, H.; Khare, P.; Singh, S.; Singh, S.P. Carbon nanomaterials integrated molecularly imprinted polymers for biological sample analysis: A critical review. Mater. Chem. Phys. 2020, 239, 121966. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Nooredeen, N.M.; El-Ghaffar, A.; Darwish, W.; Elshereafy, E.; Radwan, A.; Abbas, M. Graphene oxide with covalently attached zinc monoamino-phthalocyanine coated graphite electrode as a potentiometric platform for citrate sensing in pharmaceutical preparations. J. Solid State Electrochem. 2015, 19, 2141–2154. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.; Kharkova, A.; Kurbanaliyeva, S.; Kuznetsova, L.; Machulin, A.; Tarasov, S.; Melnikov, P.; Ponamoreva, O.; Alferov, V.; Reshetilov, A. Use of biocompatible redox-active polymers based on carbon nanotubes and modified organic matrices for development of a highly sensitive BOD biosensor. Enzym. Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Feng, Y.; Chen, H. Exploiting core–shell synergy for nanosynthesis and mechanistic investigation. Acc. Chem. Res. 2013, 46, 1636–1646. [Google Scholar] [CrossRef]
- Core, R.G.C. Classes, Properties, Synthesis Mechanisms, Characterization, and Applications/Rajib Ghosh Chaudhuri, Santanu Paria. Chem. Rev. 2012, 112, 2373. [Google Scholar]
- Singh, B.; Seddon, B.; Dempsey, E.; Redington, W.; Dickinson, C. Porous Core-Shell Platinum-Silver Nanocatalyst for the Electrooxidation of Methanol. Electroanalysis 2015, 27, 135–143. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Prokhorov, E.; Bahena, D.; Esparza, R.; Meyyappan, M. Chitosan-covered Pd@Pt core–shell nanocubes for direct electron transfer in electrochemical enzymatic glucose biosensor. ACS Omega 2017, 2, 1896–1904. [Google Scholar] [CrossRef]
- Li, M.; Wang, P.; Li, F.; Chu, Q.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of mesoporous core–shell Pd@Pt nanoparticles/amino group functionalized graphene nanocomposite. Biosens. Bioelectron. 2017, 87, 752–759. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.-D.; Wang, A.-J.; Xue, Y.; Wu, L.; Feng, J.-J. A new label-free electrochemical immunosensor based on dendritic core-shell AuPd@ Au nanocrystals for highly sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 99, 458–463. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Lv, H.; Feng, J.; Gao, Z.; Wang, P.; Dong, Y.; Liu, Q.; Zhao, Z. Sandwich-type electrochemical immunosensor based on Au@Ag supported on functionalized phenolic resin microporous carbon spheres for ultrasensitive analysis of α-fetoprotein. Biosens. Bioelectron. 2018, 106, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, Z.; Zhuo, Y.; Chai, Y.; Yuan, R. Ultrasensitive electrochemical immunosensor for carbohydrate antigen 19-9 using Au/porous graphene nanocomposites as platform and Au@Pd core/shell bimetallic functionalized graphene nanocomposites as signal enhancers. Biosens. Bioelectron. 2015, 66, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yi, H.; Yuan, Y.; Jing, P.; Chai, Y.; Yuan, R.; Wilson, G.S. An electrochemical aptasensor for thrombin using synergetic catalysis of enzyme and porous Au@Pd core–shell nanostructures for signal amplification. Biosens. Bioelectron. 2015, 64, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.A.; Shamsipur, M.; Saber, R.; Sarkar, S.; Sherkatkhameneh, N. Flow injection amperometric sandwich-type electrochemical aptasensor for the determination of adenocarcinoma gastric cancer cell using aptamer-Au@Ag nanoparticles as labeled aptamer. Electrochim. Acta 2017, 246, 1147–1154. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Taleat, Z. Synthesis and electrocatalytic effect of Ag@Pt core–shell nanoparticles supported on reduced graphene oxide for sensitive and simple label-free electrochemical aptasensor. Biosens. Bioelectron. 2015, 74, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, B.; Ji, H.; Song, Y.; Liu, J.; Peng, D.; He, L.; Zhang, Z. Aptasensor based on hierarchical core–shell nanocomposites of zirconium hexacyanoferrate nanoparticles and mesoporous mFe3O4@ mC: Electrochemical quantitation of epithelial tumor marker Mucin-1. Acs Omega 2017, 2, 6809–6818. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, X. A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Chen, L.; Ma, Z.; Zhang, W.; Liu, Z.; Cheng, Y.; Du, L.; Li, M. Bioluminescent probe for hydrogen peroxide imaging in vitro and in vivo. Anal. Chem. 2014, 86, 9800–9806. [Google Scholar] [CrossRef]
- Hang, T.; Xiao, S.; Yang, C.; Li, X.; Guo, C.; He, G.; Li, B.; Yang, C.; Chen, H.-j.; Liu, F.; et al. Hierarchical graphene/nanorods-based H2O2 electrochemical sensor with self-cleaning and anti-biofouling properties. Sens. Actuators B Chem. 2019, 289, 15–23. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, J. Environmentally friendly synthesis of Co-based zeolitic imidazolate framework and its application as H2O2 sensor. Chem. Eng. J. 2020, 392, 123690. [Google Scholar] [CrossRef]
- Shang, L.; Zeng, B.; Zhao, F. Fabrication of novel nitrogen-doped graphene–hollow AuPd nanoparticle hybrid films for the highly efficient electrocatalytic reduction of H2O2. ACS Appl. Mater. Interfaces 2015, 7, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhong, H.; Cheng, Z.; Li, X.; Zhang, A.; Li, T.; Zhang, Y.; Liu, G.; Qian, H. Magnetic Fe3O4 stacked sphere-like nanocomposite and its application as platform for H2O2 sensing. J. Electroanal. Chem. 2018, 814, 1–6. [Google Scholar] [CrossRef]
- Nagaraja, P.; Prakash, J.; Asha, S.; Bhaskara, B.; Kumar, S.A. Dibenzazepin hydrochloride as a new spectrophotometric reagent for determination of hydrogen peroxide in plant extracts. Environ. Monit. Assess. 2012, 184, 5983–5988. [Google Scholar] [CrossRef]
- Hoshino, M.; Kamino, S.; Doi, M.; Takada, S.; Mitani, S.; Yanagihara, R.; Asano, M.; Yamaguchi, T.; Fujita, Y. Spectrophotometric determination of hydrogen peroxide with osmium(VIII) and m-carboxyphenylfluorone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 814–816. [Google Scholar] [CrossRef]
- Jamil, L.A.; Faizullah, A.T.; Saleem, P.H. Flow Injection Analysis of Hydrogen Peroxide with Peroxyoxalate Chemiluminescence Detection. Sci. J. Univ. Zakho 2017, 5, 88–92. [Google Scholar] [CrossRef]
- Yu, D.; Wang, P.; Zhao, Y.; Fan, A. Iodophenol blue-enhanced luminol chemiluminescence and its application to hydrogen peroxide and glucose detection. Talanta 2016, 146, 655–661. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Wu, X.-Y.; Guan, Q.-M.; Li, H.-N. Graphene enhanced electrochemiluminescence of CdS nanocrystal for H2O2 sensing. Talanta 2010, 82, 372–376. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef]
- Xu, M.; Han, J.-M.; Wang, C.; Yang, X.; Pei, J.; Zang, L. Fluorescence ratiometric sensor for trace vapor detection of hydrogen peroxide. ACS Appl. Mater. Interfaces 2014, 6, 8708–8714. [Google Scholar] [CrossRef]
- Chang, J.; Li, H.; Hou, T.; Duan, W.; Li, F. Paper-based fluorescent sensor via aggregation induced emission fluorogen for facile and sensitive visual detection of hydrogen peroxide and glucose. Biosens. Bioelectron. 2018, 104, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Tarvin, M.; McCord, B.; Mount, K.; Sherlach, K.; Miller, M.L. Optimization of two methods for the analysis of hydrogen peroxide: High performance liquid chromatography with fluorescence detection and high performance liquid chromatography with electrochemical detection in direct current mode. J. Chromatogr. A 2010, 1217, 7564–7572. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-C.; Jin, H.-J.; Chai, X.-S. Rapid determination of hydrogen peroxide in pulp bleaching effluents by headspace gas chromatography. J. Chromatogr. A 2012, 1235, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, W.; Pan, X.; Zhao, Q.; Wei, X.; Lu, Q. High Sensitive and Selective Sensing of Hydrogen Peroxide Released from Pheochromocytoma Cells Based on Pt-Au Bimetallic Nanoparticles Electrodeposited on Reduced Graphene Sheets. Sensors 2015, 15, 2709–2722. [Google Scholar] [CrossRef]
- He, G.; Gao, F.; Li, W.; Li, P.; Zhang, X.; Yin, H.; Yang, B.; Liu, Y.; Zhang, S. Electrochemical sensing of H 2 O 2 released from living cells based on AuPd alloy-modified PDA nanotubes. Anal. Methods 2019, 11, 1651–1656. [Google Scholar] [CrossRef]
- Dai, H.; Lü, W.; Zuo, X.; Zhu, Q.; Pan, C.; Niu, X.; Liu, J.; Chen, H.; Chen, X. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells. Biosens. Bioelectron. 2017, 95, 131–137. [Google Scholar] [CrossRef]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd nanoparticles decorated N-doped graphene quantum dots@N-doped carbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Tarahomi, M.; Fayazi, M.; Ghanei-Motlagh, M.; Maleki, B. A non-enzymatic hydrogen peroxide sensor based on dendrimer functionalized magnetic graphene oxide decorated with palladium nanoparticles. Appl. Surf. Sci. 2019, 478, 87–93. [Google Scholar] [CrossRef]
- Bozkurt, S.; Tosun, B.; Sen, B.; Akocak, S.; Savk, A.; Ebeoğlugil, M.F.; Sen, F. A hydrogen peroxide sensor based on TNM functionalized reduced graphene oxide grafted with highly monodisperse Pd nanoparticles. Anal. Chim. Acta 2017, 989, 88–94. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Asadpour-Zeynali, K.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Non-enzymatic hydrogen peroxide sensor based on graphene quantum dots-chitosan/methylene blue hybrid nanostructures. Electrochim. Acta 2017, 246, 303–314. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.-W. An inkjet-printed non-enzymatic hydrogen peroxide sensor on paper. J. Electrochem. Soc. 2017, 164, B3101–B3106. [Google Scholar] [CrossRef]
- Nia, P.M.; Woi, P.M.; Alias, Y. Facile one-step electrochemical deposition of copper nanoparticles and reduced graphene oxide as nonenzymatic hydrogen peroxide sensor. Appl. Surf. Sci. 2017, 413, 56–65. [Google Scholar]
- Aparicio-Martínez, E.; Ibarra, A.; Estrada-Moreno, I.A.; Osuna, V.; Dominguez, R.B. Flexible electrochemical sensor based on laser scribed Graphene/Ag nanoparticles for non-enzymatic hydrogen peroxide detection. Sens. Actuators B Chem. 2019, 301, 127101. [Google Scholar] [CrossRef]
- Li, B.; Song, H.-Y.; Deng, Z.-P.; Huo, L.-H.; Gao, S. Novel sensitive amperometric hydrogen peroxide sensor using layered hierarchical porous α-MoO3 and GO modified glass carbon electrode. Sens. Actuators B Chem. 2019, 288, 641–648. [Google Scholar] [CrossRef]
- Agrisuelas, J.; González-Sánchez, M.-I.; Valero, E. Hydrogen peroxide sensor based on in situ grown Pt nanoparticles from waste screen-printed electrodes. Sens. Actuators B Chem. 2017, 249, 499–505. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdul Hamid, M.A.; Shamsudin, R.; Al-Khalqi, E.M.; Kar Keng, L.; Ahmed, N.M. Electrochemical hydrogen peroxide sensor based on macroporous silicon. Sensors 2018, 18, 716. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; He, H.; Shi, L.; Cai, X.; Lan, M. Pt-Pd bimetallic nanocoral modified carbon fiber microelectrode as a sensitive hydrogen peroxide sensor for cellular detection. Sens. Actuators B Chem. 2018, 260, 174–182. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sukanya, R.; Chen, S.-M.; Pandi, K.; Ho, K.-C. Synthesis and characterization of bimetallic nickel-cobalt chalcogenides (NiCoSe2, NiCo2S4, and NiCo2O4) for non-enzymatic hydrogen peroxide sensor and energy storage: Electrochemical properties dependence on the metal-to-chalcogen composition. Renew. Energy 2019, 138, 139–151. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, J.; Wu, H.; Wang, S.; Fneg, C.; Yang, S.; Ding, Y. Electrochemical hydrogen peroxide sensor based on carbon supported Cu@Pt core-shell nanoparticles. Mater. Sci. Eng. C 2017, 78, 185–190. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Hosseinifar, A. A novel amperometric hydrogen peroxide sensor based on gold nanoparticles supported on Fe3O4@polyethyleneimine. Int. J. Environ. Anal. Chem. 2020, 100, 591–601. [Google Scholar] [CrossRef]
- Han, L.; Tang, L.; Deng, D.; He, H.; Zhou, M.; Luo, L. A novel hydrogen peroxide sensor based on electrodeposited copper/cuprous oxide nanocomposites. Analyst 2019, 144, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, C.; Zhang, M. A novel electrochemical hydrogen peroxide sensor based on AuNPs/n-type GaN electrode. Chem. Lett. 2020, 49, 656–658. [Google Scholar] [CrossRef]
- Banerjee, S.; Hossain, M.F.; Slaughter, G. A Highly Sensitive Non-Enzymatic Hydrogen Peroxide Sensor based on Palladium-Gold Nanoparticles. In Proceedings of the 2020 IEEE 15th International Conference on Nano/Micro Engineered and Molecular System (NEMS), San Diego, CA, USA, 27–30 September 2020; pp. 286–289. [Google Scholar]
- Wang, Z.; Xie, F.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. High-performance non-enzyme hydrogen peroxide detection in neutral solution: Using a nickel borate nanoarray as a 3D electrochemical sensor. Chem. Eur. J. 2017, 23, 16179–16183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.J.; Kim, J.H.; Lee, G.-J. Electrochemical Detection of H2O2 Released from Prostate Cancer Cells Using Pt Nanoparticle-Decorated rGO–CNT Nanocomposite-Modified Screen-Printed Carbon Electrodes. Chemosensors 2020, 8, 63. [Google Scholar] [CrossRef]
- Oh, J.T.; Chowdhury, S.R.; Lee, T.L.; Misra, M. Synergetic influence of Au/Cu2O core-shells nanoparticle on optical, photo-electrochemical, and catalytic activities of Au/Cu2O/TiO2 nanocomposite. Dye. Pigment. 2019, 160, 936–943. [Google Scholar] [CrossRef]
- Chen, T.-M.; Xu, G.-Y.; Ren, H.; Zhang, H.; Tian, Z.-Q.; Li, J.-F. Synthesis of Au@TiO2 core–shell nanoparticles with tunable structures for plasmon-enhanced photocatalysis. Nanoscale Adv. 2019, 1, 4522–4528. [Google Scholar] [CrossRef]
- Goebl, J.; Joo, J.B.; Dahl, M.; Yin, Y. Synthesis of tailored Au@TiO2 core–shell nanoparticles for photocatalytic reforming of ethanol. Catal. Today 2014, 225, 90–95. [Google Scholar] [CrossRef]
- Sun, H.; He, Q.; Zeng, S.; She, P.; Zhang, X.; Li, J.; Liu, Z. Controllable growth of Au@TiO2 yolk–shell nanoparticles and their geometry parameter effects on photocatalytic activity. New J. Chem. 2017, 41, 7244–7252. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Chen, A.; Pu, W.; Gong, J. Influence of yolk-shell Au@TiO2 structure induced photocatalytic activity towards gaseous pollutant degradation under visible light. Appl. Catal. B Environ. 2019, 251, 57–65. [Google Scholar] [CrossRef]
- Baba, K.; Bulou, S.; Quesada-Gonzalez, M.; Bonot, S.; Collard, D.; Boscher, N.D.; Choquet, P. Significance of a noble metal nanolayer on the UV and visible light photocatalytic activity of anatase TiO2 thin films grown from a scalable PECVD/PVD approach. ACS Appl. Mater. Interfaces 2017, 9, 41200–41209. [Google Scholar] [CrossRef]
- Sun, H.; He, Q.; She, P.; Zeng, S.; Xu, K.; Li, J.; Liang, S.; Liu, Z. One-pot synthesis of Au@TiO2 yolk-shell nanoparticles with enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017, 505, 884–891. [Google Scholar] [CrossRef] [PubMed]












| Electrode | IOxi (µA) | E (mV) | IRed (µA) | E (mV) | ΔE (mV) | Rs (Ω) | Rct (Ω) | C (µF) | W (mΩ) |
|---|---|---|---|---|---|---|---|---|---|
| Bare GCE | 45.83 | 252 | −44.61 | 32 | 220 | 128.4 | 2610 | 0.49 | 0.143 |
| MWCNTs/GCE | 60.71 | 223 | −61.61 | 94 | 129 | 113.9 | 634 | 0.91 | 0.167 |
| Au@TiO2/GCE | 61.81 | 213 | −63.04 | 87 | 126 | 118.4 | 529 | 1.13 | 0.149 |
| Au@TiO2/MWCNTs/GCE | 69.77 | 228 | −69.56 | 113 | 115 | 124.8 | 281 | 1.00 | 0.266 |
| Electrode Material | Linear Range | LOD (µM) | Ref. |
|---|---|---|---|
| GO-Fe3O4-PAMAM-Pd/GCE | 0.05–160 μM | 0.01 | [48] |
| Pd/TNM@rGO | up to 12 mM | 0.0025 | [49] |
| GQDs-CS/MB/GCE | 1.0 µM–2.9 mM 2.9–11.78 mM | 0.7 | [50] |
| Paper/CNTs/AgNPs | 1 μM–700 μM | - | [51] |
| CuNPs-rGO | up to 18 mM | 601 | [52] |
| LSG-Ag | 0.1–10 mM | 7.9 | [53] |
| α-MoO3/GO/GCE | 0.92 μM–2.46 mM | 0.31 | [54] |
| PtNPs@SPCEs | 0–215 µM | 1.9 | [55] |
| MPS electrode | 10 and 5000 μM | 4.35 | [56] |
| Pt-Pd/CFME | 5–3920 µM | 0.42 | [57] |
| NiCoSe2/GCE | 0.05 to 402 µM | 0.03 | [58] |
| Cu@Pt/C | 0.50 μM–32.56 mM | 0.15 | [59] |
| Fe3O4@PEI@AuNPs-GRE | 0.2–500.0 μM | 0.07 | [60] |
| Cu/Cu2O/FTO | 0.2–2000 μM | 0.04 | [61] |
| AuNPs/n-GaN | 40 µM–1 mM | 10 | [62] |
| Pd/AuNPs | 0.5–6 mM | - | [63] |
| Ni−Bi/CC | 0.1 μM–0.5 mM | 0.00085 | [64] |
| PtNP/rGO–CNT/PtNP/SPCE | 25–1000 µM | 4.3 | [65] |
| Au@TiO2/MWCNTs/GCE | 5–200 µM and 200 µM–6 mM | 1.4 | This work |
| Interferent (10-Folds) | Recovery (%) (n = 3) |
|---|---|
| Ascorbic acid | 89.11 ± 0.50 |
| Glucose | 102.45 ± 0.87 |
| Methionine | 96.21 ± 1.23 |
| Cysteine | 105.65 ± 0.68 |
| Uric acid | 97.31 ± 0.73 |
| Sample | Added (µM) | Found in Serum (µM) (n = 3) | Recovery (%) | Found in Saliva (µM) (n = 3) | Recovery (%) |
|---|---|---|---|---|---|
| 1 | 20 | 18.02 ± 0.94 | 90.09 | 18.33 ± 0.71 | 91.63 |
| 40 | 38.10 ± 0.89 | 95.24 | 38.28 ± 0.60 | 95.71 | |
| 60 | 51.17 ± 0.70 | 85.29 | 70.74 ± 1.43 | 117.90 | |
| 2 | 20 | 20.70 ± 1.20 | 103.48 | 21.30 ± 0.87 | 106.52 |
| 40 | 37.41 ± 0.64 | 93.53 | 43.98 ± 0.69 | 109.95 | |
| 60 | 57.39 ± 0.56 | 95.65 | 63.85 ± 0.45 | 106.41 | |
| 3 | 20 | 22.11 ± 0.88 | 110.54 | 22.76 ± 1.02 | 113.80 |
| 40 | 38.10 ± 1.09 | 95.24 | 39.63 ± 0.70 | 99.08 | |
| 60 | 51.17 ± 0.79 | 85.29 | 68.30 ± 0.83 | 113.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, A.A.; Abbas, M.N.; El-Hawary, W.F.; Issa, Y.M.; Singh, B. A Core–Shell Au@TiO2 and Multi-Walled Carbon Nanotube-Based Sensor for the Electroanalytical Determination of H2O2 in Human Blood Serum and Saliva. Biosensors 2022, 12, 778. https://doi.org/10.3390/bios12100778
Saeed AA, Abbas MN, El-Hawary WF, Issa YM, Singh B. A Core–Shell Au@TiO2 and Multi-Walled Carbon Nanotube-Based Sensor for the Electroanalytical Determination of H2O2 in Human Blood Serum and Saliva. Biosensors. 2022; 12(10):778. https://doi.org/10.3390/bios12100778
Chicago/Turabian StyleSaeed, Ayman Ali, Mohammed Nooredeen Abbas, Waheed Fathi El-Hawary, Yousry Moustafa Issa, and Baljit Singh. 2022. "A Core–Shell Au@TiO2 and Multi-Walled Carbon Nanotube-Based Sensor for the Electroanalytical Determination of H2O2 in Human Blood Serum and Saliva" Biosensors 12, no. 10: 778. https://doi.org/10.3390/bios12100778