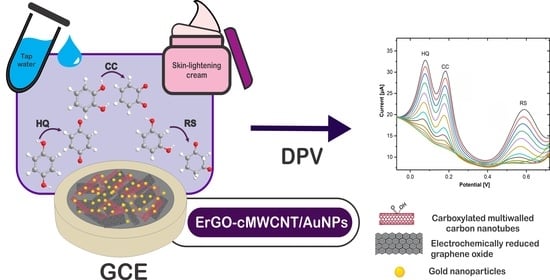
Simultaneous Detection of Dihydroxybenzene Isomers Using Electrochemically Reduced Graphene Oxide-Carboxylated Carbon Nanotubes/Gold Nanoparticles Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Materials and Reagents
2.3. GCE Modification with ErGO-cMWCNT/AuNPs Nanocomposite
2.4. Electrochemical Measurements and Real Sample Analysis
3. Results and Discussion
3.1. Materials Characterization
3.1.1. SEM
3.1.2. Raman
3.2. Electrochemical Characterization
3.3. Electrochemical Detection of Dihydroxybenzene Isomers
3.4. Effect of pH and Scan Rate
3.5. Individual and Simultaneous Determination of HQ, CC, and RS
3.6. Reproduciblity and Stability
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pifer, J.W.; Hearne, F.T.; Swanson, F.A.; O’Donoghue, J.L. Mortality study of employees engaged in the manufacture and use of hydroquinone. Int. Arch. Occup. Environ. Health 1995, 67, 267–280. [Google Scholar] [CrossRef]
- Tse, T.W. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop? J. Dermatol. Treat. 2010, 21, 272–275. [Google Scholar] [CrossRef]
- Trevors, J.T.; Basaraba, J. Toxicity of benzoquinone and hydroquinone in short-term bacterial bioassays. Bull. Environ. Contam. Toxicol. 1980, 25, 672–675. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, A.P. The toxicology of hydroquinone—Relevance to occupational and environmental exposure. Crit. Rev. Toxicol. 1999, 29, 283–330. [Google Scholar] [CrossRef]
- Hebeda, C.B.; Pinedo, F.J.; Bolonheis, S.M.; Ferreira, Z.F.; Muscará, M.N.; Teixeira, S.A.; Farsky, S.H.P. Intracellular mechanisms of hydroquinone toxicity on endotoxin-activated neutrophils. Arch. Toxicol. 2012, 86, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Karim, F.; Fakhruddin, A.N.M. Recent advances in the development of biosensor for phenol: A review. Rev. Environ. Sci. Biotechnol. 2012, 11, 261–274. [Google Scholar] [CrossRef]
- Maleki, N.; Kashanian, S.; Maleki, E.; Nazari, M. A novel enzyme based biosensor for catechol detection in water samples using artificial neural network. Biochem. Eng. J. 2017, 128, 1–11. [Google Scholar] [CrossRef]
- Barbaud, A.; Modiano, P.; Cocciale, M.; Reichert, S.; Schmutz, J.L. The topical application of resorcinol can provoke a systemic allergic reaction. Br. J. Dermatol. 1996, 135, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.S.; Delzell, E.S.; Bechtel, D.H. Toxicology review and risk assessment of resorcinol: Thyroid effects. Regul. Toxicol. Pharmacol. 2002, 36, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, H.; Li, H.; Chen, J.; Li, S.; Zheng, F. Self-template synthesis of biomass-derived 3D hierarchical N-doped porous carbon for simultaneous determination of dihydroxybenzene isomers. Sci. Rep. 2017, 7, 14985. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-F.; Li, S.-X.; Wu, Y.-J.; Chen, D.-J.; Li, Y.-H.; Zheng, F.-Y.; Yu, H.-W. Nitrogen-doped carbon spheres surface modified with in situ synthesized Au nanoparticles as electrochemical selective sensor for simultaneous detection of trace nitrophenol and dihydroxybenzene isomers. Sens. Actuators B 2016, 237, 487–494. [Google Scholar] [CrossRef]
- Tanaka, K. Resorcinol. J. Synth. Org. Chem. Jpn. 1985, 43, 262. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Rivas, G.A. Glassy carbon paste electrodes modified with polyphenol oxidase: Analytical applications. Anal. Chim. Acta 2002, 459, 43–51. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, S.; Hu, F.; Wang, C.; Yuan, R. Non-enzymatic amperometric sensor of catechol and hydroquinone using Pt-Au-organosilica@chitosan composites modified electrode. Sens. Actuators B Chem. 2012, 168, 193–199. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, Q.; Qiao, J.; Xu, Y.; Li, G. In situ synthesis of sandwich MOFs on reduced graphene oxide for electrochemical sensing of dihydroxybenzene isomers. Analyst 2019, 144, 2120–2129. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, Y.; Li, H.; Han, B.; Lin, R.; Huang, B. N-doped carbon nanotube frameworks modified electrode for the selective sensing of hydroquinone and catechol. J. Electroanal. Chem. 2020, 861, 113968. [Google Scholar] [CrossRef]
- Lu, J.Y.; Yu, Y.S.; Chen, T.B.; Chang, C.F.; Tamulevičius, S.; Erts, D.; Wu, K.C.W.; Gu, Y. Fabrication of an extremely cheap poly(3,4-ethylenedioxythiophene) modified pencil lead electrode for effective hydroquinone sensing. Polymers 2021, 13, 343. [Google Scholar] [CrossRef]
- Yang, J.; Deng, S.; Lei, J.; Ju, H.; Gunasekaran, S. Electrochemical synthesis of reduced graphene sheet-AuPd alloy nanoparticle composites for enzymatic biosensing. Biosens. Bioelectron. 2011, 29, 159–166. [Google Scholar] [CrossRef]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-iron selenides embedded in porous carbon nanofibers for simultaneous electrochemical detection of trace of hydroquinone, catechol and resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef]
- Chen, T.-W. Simultaneous Determination of Dihydroxybenzene Isomers using Glass Carbon Electrode Modified with 3D CNT-graphene Decorated with Au Nanoparticles. Int. J. Electrochem. Sci. 2019, 14, 7037–7046. [Google Scholar] [CrossRef]
- Butwong, N.; Kunawong, T.; Luong, J.H.T. Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan. Nanomaterials 2020, 10, 1583. [Google Scholar] [CrossRef]
- Torrinha, Á.; Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Correia, A.N.; Lima-Neto, P.; Morais, S. Application of Nanostructured Carbon-Based Electrochemical (Bio)Sensors for Screening of Emerging Pharmaceutical Pollutants in Waters and Aquatic Species: A Review. Nanomaterials 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Chen, S.M.; Lou, B.S. Three dimensional graphene oxide-carbon nanotubes and graphene-carbon nanotubes hybrids. Int. J. Electrochem. Sci. 2013, 8, 11641–11660. [Google Scholar]
- Dang, V.T.; Nguyen, D.D.; Cao, T.T.; Le, P.H.; Tran, D.L.; Phan, N.M.; Nguyen, V.C. Recent trends in preparation and application of carbon nanotube–graphene hybrid thin films. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 033002. [Google Scholar] [CrossRef] [Green Version]
- Filik, H.; Avan, A.A. Review on applications of carbon nanomaterials for simultaneous electrochemical sensing of environmental contaminant dihydroxybenzene isomers. Arab. J. Chem. 2020, 13, 6092–6105. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Jin, X.; Zhang, L. Electrochemical determination of estrogenic compound bisphenol F in food packaging using carboxyl functionalized multi-walled carbon nanotubes modified glassy carbon electrode. Food Chem. 2014, 157, 464–469. [Google Scholar] [CrossRef]
- Carneiro, P.; Morais, S.; Pereira, M.C. Nanomaterials towards Biosensing of Alzheimer’s Disease Biomarkers. Nanomaterials 2019, 9, 1663. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hoa, L.M.T. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4. Diam. Relat. Mater. 2018, 89, 43–51. [Google Scholar] [CrossRef]
- Yu, H.; Li, R.; Song, K. Amperometric determination of nitrite by using a nanocomposite prepared from gold nanoparticles, reduced graphene oxide and multi-walled carbon nanotubes. Microchim. Acta 2019, 186, 624. [Google Scholar] [CrossRef]
- Kıranşan, K.D.; Topçu, E. Free-standing and Flexible MoS2/rGO Paper Electrode for Amperometric Detection of Folic Acid. Electroanalysis 2018, 30, 810–818. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Avan, A.A.; Filik, H. Simultaneous electrochemical sensing of dihydroxybenzene isomers at multi-walled carbon nanotubes aerogel/gold nanoparticles modified graphene screen-printed electrode. J. Electroanal. Chem. 2020, 878, 114682. [Google Scholar] [CrossRef]
- Yang, S.; Yang, M.; Yao, X.; Fa, H.; Wang, Y.; Hou, C. A zeolitic imidazolate framework/carbon nanofiber nanocomposite based electrochemical sensor for simultaneous detection of co-existing dihydroxybenzene isomers. Sens. Actuators B Chem. 2020, 320, 128294. [Google Scholar] [CrossRef]
- Laurila, T.; Sainio, S.; Caro, M.A. Hybrid carbon based nanomaterials for electrochemical detection of biomolecules. Prog. Mater. Sci. 2017, 88, 499–594. [Google Scholar] [CrossRef]
- Bi, H.; Li, Y.; Liu, S.; Guo, P.; Wei, Z.; Lv, C.; Zhang, J.; Zhao, X.S. Carbon-nanotube-modified glassy carbon electrode for simultaneous determination of dopamine, ascorbic acid and uric acid: The effect of functional groups. Sens. Actuators B Chem. 2012, 171–172, 1132–1140. [Google Scholar] [CrossRef]
- Moraes, F.C.; Cabral, M.F.; Mascaro, L.H.; MacHado, S.A.S. The electrochemical effect of acid functionalisation of carbon nanotubes to be used in sensors development. Surf. Sci. 2011, 605, 435–440. [Google Scholar] [CrossRef]
- Goulart, L.A.; De Moraes, F.C.; Mascaro, L.H. Influence of the different carbon nanotubes on the development of electrochemical sensors for bisphenol A. Mater. Sci. Eng. C 2016, 58, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-Y.; Hsiao, M.-C.; Liao, S.-H.; Liu, P.-I.; Tsai, H.-M.; Ma, C.-C.M.; Pu, N.-W.; Ger, M.-D. Preparation of graphene/multi-walled carbon nanotube hybrid and its use as photoanodes of dye-sensitized solar cells. Carbon 2011, 49, 3597–3606. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, J.; Han, D.; Zhao, S.; Zhu, R.; Cui, G. A three-electrode integrated electrochemical platform based on nanoporous gold for the simultaneous determination of hydroquinone and catechol with high selectivity. Analyst 2021, 146, 232–243. [Google Scholar] [CrossRef]
- Huang, R.; Liao, D.; Chen, S.; Yu, J.; Jiang, X. A strategy for effective electrochemical detection of hydroquinone and catechol: Decoration of alkalization-intercalated Ti3C2 with MOF-derived N-doped porous carbon. Sens. Actuators B Chem. 2020, 320, 128386. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Zheng, X.Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef]
- Edris, N.M.M.A.; Sulaiman, Y. Ultrasensitive voltammetric detection of benzenediol isomers using reduced graphene oxide-azo dye decorated with gold nanoparticles. Ecotoxicol. Environ. Saf. 2020, 203, 111026. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, M.; Karikalan, N.; Chen, S.-M.; Cheng, Y.-H.; Karuppiah, C. Electrochemical preparation of activated graphene oxide for the simultaneous determination of hydroquinone and catechol. J. Colloid Interface Sci. 2017, 500, 54–62. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Aragó, M.; Ariño, C.; Dago, À.; Díaz-Cruz, J.M.; Esteban, M. Simultaneous determination of hydroquinone, catechol and resorcinol by voltammetry using graphene screen-printed electrodes and partial least squares calibration. Talanta 2016, 160, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, X.; Zhang, S.; Yang, L.; Liu, M.; Zhang, Y.; Yao, S. Ultrasensitive and simultaneous detection of hydroquinone, catechol and resorcinol based on the electrochemical co-reduction prepared Au-Pd nanoflower/reduced graphene oxide nanocomposite. Electrochim. Acta 2017, 231, 677–685. [Google Scholar] [CrossRef]
- Zhang, C.; Han, M.; Yu, L.; Qu, L.; Li, Z. Fabrication an electrochemical sensor based on composite of Cu-TCPP nanosheets and PSS functionalized graphene for simultaneous and sensitive determination of dihydroxybenzene isomers. J. Electroanal. Chem. 2021, 890, 115232. [Google Scholar] [CrossRef]
- Yang, S.; Yang, M.; Liu, Q.; Wang, X.; Fa, H.; Wang, Y.; Hou, C. An Ultrasensitive Electrochemical Sensor Based on Multiwalled Carbon Nanotube@Reduced Graphene Oxide Nanoribbon Composite for Simultaneous Determination of Hydroquinone, Catechol and Resorcinol. J. Electrochem. Soc. 2019, 166, B547–B553. [Google Scholar] [CrossRef]
- Deng, M.; Lin, S.; Bo, X.; Guo, L. Simultaneous and sensitive electrochemical detection of dihydroxybenzene isomers with UiO-66 metal-organic framework/mesoporous carbon. Talanta 2017, 174, 527–538. [Google Scholar] [CrossRef]











| Analyte | Potential (V) | Linear Range (μM) | Sensitivity (μA.mM.cm−2) | LOD (μM) |
|---|---|---|---|---|
| HQ | 0.073 | 1.2–26, 26–170 | 1962, 854 | 0.39 |
| CC | 0.18 | 1.2–26, 26–170 | 1904, 922 | 0.55 |
| RS | 0.58 | 2.5–52, 52–342 | 488, 344 | 0.61 |
| Modified Electrode | Real Sample | Linear Range (µM) | Detection Limit (µM) | References | ||||
|---|---|---|---|---|---|---|---|---|
| HQ | CC | RS | HQ | CC | RS | |||
| 3D CNT-Gr/AuNPs/GCE | Tap, river water | 2–80 | 2–80 | 2–80 | 0.8 | 0.95 | 0.1 | [20] |
| AuNPs/NfCAG/Gr-SPE | Tap, mineral water | 0.2–75 | 0.2–50 | 0.2–125 | 0.014 | 0.017 | 0.05 | [33] |
| ERGO-poly(PR)/AuNPs/GCE | Wastewater and cosmetic sample | 0.1–90 | 0.4–90 | 4–350 | 0.053 | 0.053 | 0.079 | [43] |
| Gr/SPE | Tap water | 1–50 | 1–50 | 1–50 | 2.7 | 1.7 | 2.4 | [46] |
| Au@PdNF/RGO/GCE | Tap, river, and lake water | 1.6–100 | 2.5–100 | 2.0–100 | 0.5 | 0.8 | 0.7 | [47] |
| PSS-Gr@Cu-TCPP/GCE | Lake, tap water | 1–200 | 0.08–120 | 5–100 | 1 | 0.08 | 5 | [48] |
| MWCNT@rGONR/GCE | Tap, river water | 15–921 | 15–1101 | 15–1301 | 3.89 | 1.73 | 5.77 | [49] |
| UiO-66/MC-3/GCE | Tap, lake water | 0.5–100 | 0.4–100 | 30–400 | 0.056 | 0.072 | 3.51 | [50] |
| GCE/ErGO-cMWCNT/AuNPs | Tap water, skin-lightening cream | 1.2–170 | 1.2–170 | 2.5–342 | 0.39 | 0.54 | 0.61 | This work |
| Scheme 3. | Included (μM) | Spiked (μM) | Found (μM) | Recovery (%) | % RSD (n = 3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tap water | HQ | CC | RS | HQ | CC | RS | HQ | CC | RS | HQ | CC | RS | |
| - | 0.05 | 0.05 | 0.1 | 0.056 | 0.052 | 0.105 | 113.64 | 105.40 | 101.58 | 8.97 | 3.06 | 4.75 | |
| - | 0.1 | 0.1 | 0.2 | 0.11 | 0.106 | 0.22 | 110.46 | 106.08 | 110.09 | 4.42 | 1.14 | 3.21 | |
| - | 0.15 | 0.15 | 0.3 | 0.157 | 0.157 | 0.321 | 104.70 | 104.88 | 107.18 | 8.54 | 3.46 | 5.22 | |
| Skin-lightening cream | 0.111 HQ | - | 0.11 | 0.22 | 0.114 | 0.110 | 0.253 | 103.09 | 100.50 | 105.09 | 1.92 | 6.12 | 1.09 |
| 0.14 HQ | - | 0.14 | 0.28 | 0.150 | 0.145 | 0.286 | 107.26 | 103.58 | 102.41 | 2.38 | 5.47 | 7.19 | |
| 0.17 HQ | - | 0.17 | 0.34 | 0.173 | 0.169 | 0.344 | 101.79 | 99.59 | 101.179 | 4.12 | 0.95 | 0.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Aragón, A.; Dominguez, R.B.; Zaragoza-Contreras, E.A. Simultaneous Detection of Dihydroxybenzene Isomers Using Electrochemically Reduced Graphene Oxide-Carboxylated Carbon Nanotubes/Gold Nanoparticles Nanocomposite. Biosensors 2021, 11, 321. https://doi.org/10.3390/bios11090321
Domínguez-Aragón A, Dominguez RB, Zaragoza-Contreras EA. Simultaneous Detection of Dihydroxybenzene Isomers Using Electrochemically Reduced Graphene Oxide-Carboxylated Carbon Nanotubes/Gold Nanoparticles Nanocomposite. Biosensors. 2021; 11(9):321. https://doi.org/10.3390/bios11090321
Chicago/Turabian StyleDomínguez-Aragón, Angélica, Rocio B. Dominguez, and Erasto Armando Zaragoza-Contreras. 2021. "Simultaneous Detection of Dihydroxybenzene Isomers Using Electrochemically Reduced Graphene Oxide-Carboxylated Carbon Nanotubes/Gold Nanoparticles Nanocomposite" Biosensors 11, no. 9: 321. https://doi.org/10.3390/bios11090321