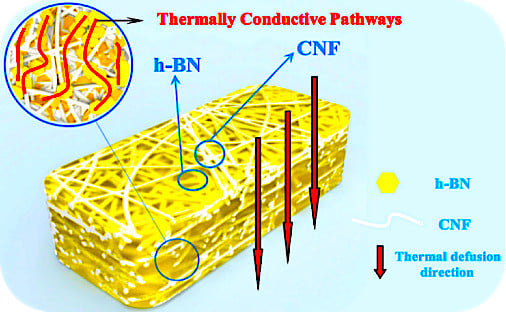
Aerogel Perfusion-Prepared h-BN/CNF Composite Film with Multiple Thermally Conductive Pathways and High Thermal Conductivity
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Cellulose-Nano Fiber (CNF)
2.3. Exfoliation of Hexagonal Boron Nitride (h-BN)
2.4. Preparation of CNF Aerogel
2.5. h-BN/CNF Composite Films Preparation
2.5.1. Preparation of h-BN/CNF Blended Composite Film
2.5.2. Preparation of h-BN/CNF Aerogel Perfusion Composite Film
2.6. Characterization
3. Results and Discussion
3.1. Preparation of h-BN/CNF Composite Film
3.2. Thermal Stability of h-BN/CNF Aerogel Perfusion Composite Films
3.3. Thermal Conductivity and Electrical Properties of h-BN/CNF Composite Films
3.4. Thermal Management Capability of the h-BN/CNF Aerogel Perfusion Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Zhang, M.; Ji, J.; Pang, X.; Liu, R. Three-Dimensional Heterostructured Reduced Graphene Oxide-Hexagonal Boron Nitride-Stacking Material for Silicone Thermal Grease with Enhanced Thermally Conductive Properties. Nanomaterials 2019, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P. Highly Thermally conductive fluorinated graphene films with superior electrical insulation and mechanical flexibility. ACS Appl. Mater. Int. 2019, 11, 21946–21954. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J.; Liu, B.; Wu, J.; Cheng, H.M.; Kang, F. Two-dimensional materials for thermal management applications. Joule 2018, 2, 442–463. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected BN nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Bian, H.; Wu, W.; Xiao, H.; Dai, H. Thermally Conductive and Electrical Insulation BNNS/CNF Aerogel Nano-Paper. Polymers 2019, 11, 660. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.B.; Wong, C.P. A combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Gadinski, M.R.; Zhang, S.; Zhang, G.; Li, H.U. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 2015, 523, 576–579. [Google Scholar] [CrossRef]
- Song, W.L.; Wang, W.; Veca, L.M.; Kong, C.Y.; Cao, M.S.; Wang, P.; Meziani, M.J.; Qian, H.; Croy, G.E.; Cao, L.; et al. Polymer/carbon nanocomposites for enhanced thermal transport properties–carbon nanotubes versus graphene sheets as nanoscale fillers. J. Mater. Chem. 2012, 22, 17133–17139. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Z.; Luo, W.; Han, X.; Jang, S.H.; Dai, J.; Yang, B.; Hu, L. Thermally conductive, electrical insulating, optically transparent bi-layer nanopaper. ACS Appl. Mater. Interfaces 2016, 8, 28838–28843. [Google Scholar] [CrossRef]
- Ikawa, M.; Yamada, T.; Matsui, H.; Minemawari, H.; Tsutsumi, J.Y.; Horii, Y.; Chikamatsu, M.; Azumi, R.; Kumai, R.; Hasegawa, T. Simple push coating of polymer thin-film transistors. Nat. Commun. 2012, 3, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, U.; Sejfić, M.; Kang, M.J.; Takimiya, K.; Zaki, T.; Letzkus, F.; Burghartz, J.N.; Weber, E.; Klauk, H. Flexible Low-Voltage Organic Complementary Circuits: Finding the Optimum Combination of Semiconductors and Monolayer Gate Dielectrics. Adv. Mater. 2015, 27, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H.; et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.A.; Rai, A.; Glynn, E.; Morgan, P.; Moore, A.L.; Youngblood, J.P. Superior, processing-dependent thermal conductivity of cellulose Nanocrystal-Poly (vinyl alcohol) composite films. Polymer 2019, 164, 17–25. [Google Scholar] [CrossRef]
- Suh, D.; Moon, C.M.; Kim, D.; Baik, S. Ultrahigh thermal conductivity of interface materials by silver-functionalized carbon nanotube phonon conduits. Adv. Mater. 2016, 28, 7220–7227. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Construction of 3D skeleton for polymer composites achieving a high thermal conductivity. Small 2018, 14, 1704044. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Huang, X.; Zhu, Y.; Chen, J.; Jiang, P. Boron nitride nanosheets endow the traditional dielectric polymer composites with advanced thermal management capability. Compos. Sci. Technol. 2019, 177, 88–95. [Google Scholar] [CrossRef]
- Putz, B.; Völker, B.; Semprimoschnig, C.; Cordill, M.J. Influence of extreme thermal cycling on metal-polymer interfaces. Microelectron. Eng. 2017, 167, 17–22. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Investigation of polyamide 11 embrittlement during oxidative degradation. Polymer 2016, 82, 49–56. [Google Scholar] [CrossRef]
- Gauthier, E.; Nikolić, M.; Truss, R.; Laycock, B.; Halley, P. Effect of soil environment on the photo-degradation of polyethylene films. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Fofana, I.; Hemmatjou, H.; Farzaneh, M. Low temperature and moisture effects on polarization and depolarization currents of oil-paper insulation. Electr. Power Syst. Res. 2010, 80, 91–97. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, P.T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Lao, J.; Xie, H.; Shi, Z.; Li, G.; Li, B.; Hu, G.H.; Yang, Q.; Xiong, C. Flexible regenerated cellulose/boron nitride nanosheet high-temperature dielectric nanocomposite films with high energy density and breakdown strength. ACS Sustain. Chem. Eng. 2018, 6, 151–7158. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Liu, C.; Fan, S.; Zhang, J.; Ding, X.; Golberg, D. Thermal conductivity of nanostructured boron nitride materials. J. Phys. Chem. B 2006, 110, 10354–10357. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, S.; Zhu, M.; Yang, K.; Jiang, P.; Bando, Y.; Golberg, D.; Zhi, C. Thermally conductive, electrically insulating and melt-processable polystyrene/boron nitride nanocomposites prepared by in situ reversible addition fragmentation chain transfer polymerization. Nanotechnology 2014, 26, 015705. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, J.; Wang, J.; Zhu, Y.; Jiang, L. Bioinspired hierarchical alumina–graphene oxide–poly (vinyl alcohol) artificial nacre with optimized strength and toughness. ACS Appl. Mater. Interfaces 2015, 7, 9281–9286. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, Y.; Qin, S.; Liu, D.; Wang, X.; Hu, X.; Li, J.; Wang, X.; Bando, Y.; Golberg, D.; et al. BN Nanosheet/polymer films with highly anisotropic thermal conductivity for thermal management applications. ACS Appl. Mater. Interfaces 2017, 9, 43163–43170. [Google Scholar] [CrossRef]
- Zha, X.J.; Yang, J.; Pu, J.H.; Feng, C.P.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Yang, W. Enhanced Thermal Conductivity and Balanced Mechanical Performance of PP/BN Composites with 1 vol% Finely Dispersed MWCNTs Assisted by OBC. Adv. Mater. Interfaces 2019, 6, 1900081. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Sun, B.; Jiang, P. Highly Thermally Conductive Yet Electrically Insulating Polymer/Boron Nitride Nanosheets Nanocomposite Films for Improved Thermal Management Capability. ACS Nano 2018, 13, 337–345. [Google Scholar] [CrossRef]
- Feng, C.P.; Wan, S.S.; Wu, W.C.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Chen, J.; Yang, W. Electrically insulating, layer structured SiR/GNPs/BN thermal management materials with enhanced thermal conductivity and breakdown voltage. Compos. Sci. Technol. 2018, 167, 456–462. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, C.; Tang, Y.; Zhang, X.; Zheng, K.; Tian, X. Preparation and properties of boron nitride nanosheets/cellulose nanofiber shear-oriented films with high thermal conductivity. Ceram. Int. 2019, 45, 12965–12974. [Google Scholar] [CrossRef]
- Diaham, S.; Zelmat, S.; Locatelli, M.L.; Dinculescu, S.; Decup, M.; Lebey, T. Dielectric breakdown of polyimide films: Area, thickness and temperature dependence. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 18–27. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Kim, K.; Ju, H.; Kim, J. Surface modification of BN/Fe3O4 hybrid particle to enhance interfacial affinity for high thermal conductive material. Polymer 2016, 91, 74–80. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Wu, Y.; Liu, X.; Kim, J.K. Effect of functionalization on thermal conductivities of graphene/epoxy composites. Carbon 2016, 108, 412–422. [Google Scholar] [CrossRef]
- Khalil, H.A.; Bhat, A.H.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Yang, W.; Bian, H.; Jiao, L.; Wu, W.; Deng, Y.; Dai, H. High wet-strength, thermally stable and transparent TEMPO-oxidized cellulose nanofibril film via cross-linking with poly-amide epichlorohydrin resin. RSC Adv. 2017, 7, 31567–31573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, T.; Liu, Z.; Wang, Q. Facile fabrication of tough photocrosslinked polyvinyl alcohol hydrogels with cellulose nanofibrils reinforcement. Polymer 2019, 173, 103–109. [Google Scholar] [CrossRef]
- Xiao, F.; Naficy, S.; Casillas, G.; Khan, M.H.; Katkus, T.; Jiang, L.; Liu, H.; Li, H.; Huang, Z. Edge-Hydroxylated Boron Nitride Nanosheets as an Effective Additive to Improve the Thermal Response of Hydrogels. Adv. Mater. 2015, 27, 7196–7203. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Liu, J.; He, Q.; Meng, Y.; Cao, L.; Sun, Y.P.; Chen, J.; Lu, F. Aqueous compatible boron nitride nanosheets for high-performance hydrogels. Nanoscale 2016, 8, 4260–4266. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, X.; Gogoi, P.; Bian, H.; Dai, H. Highly transparent and thermally stable cellulose nanofibril films functionalized with colored metal ions for Ultraviolet blocking activities. Carbohydr. Polym. 2019, 213, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Chang, T.H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Li, H.; Tay, R.Y.; Sun, B.; Tsang, S.H.; Cometto, O.; Lin, J.; Teo, E.H.T.; Tok, A.I.Y. Biocompatible hydroxylated boron nitride nanosheets/poly (vinyl alcohol) interpenetrating hydrogels with enhanced mechanical and thermal responses. ACS Nano 2017, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Bian, H.; Gao, T.; Jiang, F.; Kierzewski, I.M.; Wang, Y.; Yao, Y.; Chen, L.; Shao, Z.; Zhu, J.Y.; et al. Thermally stable cellulose nanocrystals toward high-performance 2D and 3D nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 28922–28929. [Google Scholar] [CrossRef]
- Agari, Y.; Ueda, A.; Nagai, S. Thermal conductivity of a polymer composite. J. Appl. Polym. Sci. 1993, 49, 1625–1634. [Google Scholar] [CrossRef]
- Nan, C.W.; Liu, G.; Lin, Y.; Li, M. Interface effect on thermal conductivity of carbon nanotube composites. Appl. Phys. Lett. 2004, 85, 3549–3551. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Li, W.; Qiu, S.; Zhu, C.; Wei, X.; Chen, M.; Liu, C.; Liao, S.; Gong, Y.; et al. Ultrahigh thermal conductivity of assembled aligned multilayer graphene/epoxy composite. Chem. Mater. 2014, 26, 4459–4465. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Yao, Y.; Sun, R.; Xu, J.; Wong, C.P. Silver nanoparticle-deposited boron nitride nanosheets as fillers for polymeric composites with high thermal conductivity. Sci. Rep. 2016, 6, 19394. [Google Scholar] [CrossRef]
- Fu, C.; Yan, C.; Ren, L.; Zeng, X.; Du, G.; Sun, R.; Xu, J.; Wong, C.P. Improving thermal conductivity through welding boron nitride nanosheets onto silver nanowires via silver nanoparticles. Compos. Sci. Technol. 2019, 177, 118–126. [Google Scholar] [CrossRef]







| Sample | h-BN (g) | h-BN Loading (wt %) | h-BN Loading (vol %) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 0.0084 | 4.79 | 1.59 |
| 3 | 0.0167 | 9.09 | 3.18 |
| 4 | 0.0251 | 13.07 | 4.78 |
| 5 | 0.0334 | 16.67 | 6.33 |
| 6 | 0.0418 | 20.02 | 7.92 |
| 7 | 0.0501 | 23.08 | 9.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, Z.; Jiao, L.; Bian, H.; Yang, W.; Wu, W.; Xiao, H.; Dai, H. Aerogel Perfusion-Prepared h-BN/CNF Composite Film with Multiple Thermally Conductive Pathways and High Thermal Conductivity. Nanomaterials 2019, 9, 1051. https://doi.org/10.3390/nano9071051
Wang X, Yu Z, Jiao L, Bian H, Yang W, Wu W, Xiao H, Dai H. Aerogel Perfusion-Prepared h-BN/CNF Composite Film with Multiple Thermally Conductive Pathways and High Thermal Conductivity. Nanomaterials. 2019; 9(7):1051. https://doi.org/10.3390/nano9071051
Chicago/Turabian StyleWang, Xiu, Zhihuai Yu, Liang Jiao, Huiyang Bian, Weisheng Yang, Weibing Wu, Huining Xiao, and Hongqi Dai. 2019. "Aerogel Perfusion-Prepared h-BN/CNF Composite Film with Multiple Thermally Conductive Pathways and High Thermal Conductivity" Nanomaterials 9, no. 7: 1051. https://doi.org/10.3390/nano9071051