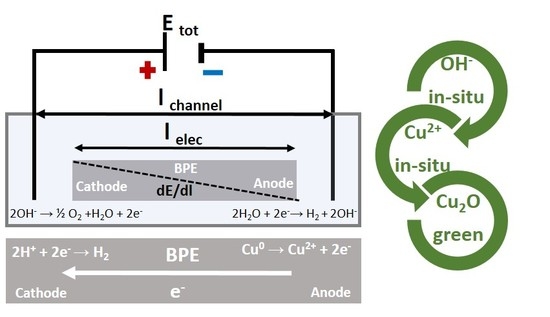
Nanostructured Cu2O Synthesized via Bipolar Electrochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.2.1. X-Ray Diffraction (XRD)
2.2.2. X-Ray Photoelectron Spectroscopy (XPS)
2.2.3. Transmission Electron/Scanning Electron Microscopy (TEM/SEM)
2.2.4. UV–Vis Diffusive Reflectance
2.2.5. Photoluminescence
2.2.6. Electrochemical Characterization
2.2.7. Fabrication of Cu2O Electrode
2.2.8. Synthesis Procedure
3. Results
3.1. Structural Analysis of Cu2O
3.2. Morphology
3.3. X-Ray Photoelectron Spectroscopy (XPS): Surface Chemistry
3.4. Optical Studies: Diffusive-Reflectance UV–Vis and Kubelka–Munk Analysis
3.5. Mott–Schottky Analysis: Flat-Band Potential and Carrier Concentration
3.6. Photo-Electrochemistry: Carrier Lifetime and Photocurrent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, S.; Cao, C.; Usman, Z.; Nabi, G.; Butt, F.K.; Mahmood, K.; Ali, A.; Arshad, M.I.; Amin, N. Effect of films morphology on the performance of Cu2O PEC solar cells. Optik 2018, 172, 72–78. [Google Scholar] [CrossRef]
- Deo, M.; Ogale, S. Crystal Facet Control for the Stability of p-Cu2O Nanoneedles as Photocathode for Photoelectrochemical Activity. Mater. Today Proc. 2018, 5, 23482–23489. [Google Scholar] [CrossRef]
- Shyamal, S.; Hajra, P.; Mandal, H.; Bera, A.; Sariket, D.; Satpati, A.K.; Malashchonak, M.V.; Mazanik, A.V.; Korolik, O.V.; Kulak, A.I.; et al. Eu modified Cu2O thin films: Significant enhancement in efficiency of photoelectrochemical processes through suppression of charge carrier recombination. Chem. Eng. J. 2018, 335, 676–684. [Google Scholar] [CrossRef]
- Baek, M.; Zafar, M.; Kim, S.; Kim, D.-H.; Jeon, C.-W.; Lee, J.; Yong, K. Enhancing Durability and Photoelectrochemical Performance of the Earth Abundant Ni–Mo/TiO2/CdS/CIGS Photocathode under Various pH Conditions. ChemSusChem 2018, 11, 3679–3688. [Google Scholar] [CrossRef]
- Yang, Y.; Han, J.; Ning, X.; Su, J.; Shi, J.; Cao, W.; Xu, W. Photoelectrochemical stability improvement of cuprous oxide (Cu2O) thin films in aqueous solution. Int. J. Energy Res. 2016, 40, 112–123. [Google Scholar] [CrossRef]
- Wong, T.K.S.; Zhuk, S.; Masudy-Panah, S.; Dalapati, G.K. Current Status and Future Prospects of Copper Oxide Heterojunction Solar Cells. Materials 2016, 9, 271. [Google Scholar] [CrossRef]
- Dhaouadi, M.; Jlassi, M.; Sta, I.; Miled, I. Ben; Mousdis, G.; Kompitsas, M.; Dimassi, W. Physical Properties of Copper Oxide Thin Films Prepared by Sol–Gel Spin–Coating Method. Am. J. Phys. Appl. 2018, 6, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Arshadi-Rastabi, S.; Moghaddam, J.; Eskandarian, M.R. Synthesis, characterization and stability of Cu2O nanoparticles produced via supersaturation method considering operational parameters effect. J. Ind. Eng. Chem. 2015, 22, 34–40. [Google Scholar] [CrossRef]
- Finney, E.E.; Finke, R.G. Nanocluster nucleation and growth kinetic and mechanistic studies: A review emphasizing transition-metal nanoclusters. J. Colloid Interface Sci. 2008, 317, 351–374. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.C.; Xu, P.; McCool, N.S.; Li, L.; Wang, W.; Mallouk, T.E. Visible-light controlled catalytic Cu2O–Au micromotors. Nanoscale 2017, 9, 75–78. [Google Scholar] [CrossRef]
- Abhilash, M.R.; Akshatha, G.; Srikantaswamy, S. Photocatalytic dye degradation and biological activities of the Fe2O3/Cu2O nanocomposite. RSC Adv. 2019, 9, 8557–8568. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, S.; Landarani-Isfahani, A.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Mono- and multifold C–C coupling reactions catalyzed by a palladium complex encapsulated in MIL-Cr as a three dimensional nano reactor. RSC Adv. 2016, 6, 92463–92472. [Google Scholar] [CrossRef]
- Keßler, M.T.; Robke, S.; Sahler, S.; Prechtl, M.H.G. Ligand-free copper(I) oxide nanoparticle-catalysed amination of aryl halides in ionic liquids. Catal. Sci. Technol. 2014, 4, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Pal, R.; Roy, M.; Chatterjee, N.; Sarkar, S.; Sen, A.K. Nanodomain cubic copper (I) oxide as reusable catalyst for the synthesis of amides by amidation of aryl halides with isocyanides Swarbhanu. Tetrahedron Lett. 2015, 56, 623–626. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Sasaki, T.; Bhanage, B.M. A facile and rapid route for the synthesis of Cu/Cu2O nanoparticles and their application in the Sonogashira coupling reaction of acyl chlorides with terminal alkynes. Catal. Sci. Technol. 2014, 4, 4274–4280. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, M.; Bhalla, V. Aggregates of the pentacenequinone derivative as reactors for the preparation of Ag@Cu2O core–shell NPs: An active photocatalyst for Suzuki and Suzuki type coupling reactions. Chem. Commun. 2015, 51, 12529–12532. [Google Scholar] [CrossRef]
- Hu, X.E. Nucleophilic ring opening of aziridines. Tetrahedron 2004, 60, 2701–2743. [Google Scholar] [CrossRef]
- Yao, Z.; Wei, X. Amidation of Aryl Halides Catalyzed by the Efficient and Recyclable Cu2O Nanoparticles. Chin. J. Chem. 2010, 28, 2260–2268. [Google Scholar] [CrossRef]
- Pasini, D. The Click Reaction as an Efficient Tool for the Construction of Macrocyclic Structures. Molecules 2013, 18, 9512–9530. [Google Scholar] [CrossRef]
- Kim, A.; Muthuchamy, N.; Yoon, C.; Joo, S.H.; Park, K.H. MOF-Derived Cu@Cu2O Nanocatalyst for Oxygen Reduction Reaction and Cycloaddition Reaction. Nanomaterials 2018, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Mateo, D.; Albero, J.; Garcia, H. Photoassisted methanation using Cu2O nanoparticles supported on graphene as a photocatalyst. Energy Environ. Sci. 2017, 10, 2392–2400. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yuan, L.; Li, S.-H.; Tang, Z.-R.; Xu, Y.-J. One-dimensional copper-based heterostructures toward photo-driven reduction of CO2 to sustainable fuels and feedstocks. J. Mater. Chem. A 2019, 7, 8676–8689. [Google Scholar] [CrossRef]
- Munir, S.; Varzeghani, A.R.; Kaya, S. Electrocatalytic reduction of CO2 to produce higher alcohols. Sustain. Energy Fuels 2018, 2, 2532–2541. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Chen, G.; Zhang, H.; Chen, Y. Synthesis of Cu2O nano-whiskers by a novel wet-chemical route. Mater. Lett. 2008, 62, 886–888. [Google Scholar] [CrossRef]
- Pathan, S.; Mishra, H.; Kachwala, S.; Nadar, S.; Marolikar, D.M.; Jha, M. Green Synthesis and Characterization of Cuprous Oxide Nanoparticles using Tea Extract. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.; Hu, M.; Liu, S.; Zhu, Y.; Cao, M. Electrochemical synthesis and the gas-sensing properties of the Cu2O nanofilms/porous silicon hybrid structure. Sens. Actuators B Chem. 2016, 223, 626–633. [Google Scholar] [CrossRef]
- Liu, C.; Chang, Y.-H.; Chen, J.; Feng, S.-P. Electrochemical Synthesis of Cu2O Concave Octahedrons with High-Index Facets and Enhanced Photoelectrochemical Activity. ACS Appl. Mater. Interfaces 2017, 9, 39027–39033. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Roshani, R. A green synthesis of copper oxide nanoparticles by mechanochemical method. Curr. Chem. Lett. 2014, 3, 215–220. [Google Scholar] [CrossRef]
- Khayati, G.R.; Nourafkan, E.; Karimi, G.; Moradgholi, J. Synthesis of cuprous oxide nanoparticles by mechanochemical oxidation of copper in high planetary energy ball mill. Adv. Powder Technol. 2013, 24, 301–305. [Google Scholar] [CrossRef]
- Yadav, S.; Jain, A.; Malhotra, P. A review on the sustainable routes for the synthesis and applications of cuprous oxide nanoparticles and their nanocomposites. Green Chem. 2019, 21, 937–955. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X. Electrochemical synthesis of copper nanoparticles using cuprous oxide as a precursor in choline chloride–urea deep eutectic solvent: Nucleation and growth mechanism. Phys. Chem. Chem. Phys. 2014, 16, 27088–27095. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, M.; Gambirasi, A.; Ugo, P. Closed Bipolar Electrochemistry for the Low-Potential Asymmetrical Functionalization of Micro- and Nanowires. ChemElectroChem 2016, 3, 450–456. [Google Scholar] [CrossRef]
- Fosdick, S.E.; Knust, K.N.; Scida, K.; Crooks, R.M. Bipolar Electrochemistry. Angew. Chem. Int. Ed. 2013, 52, 10438–10456. [Google Scholar] [CrossRef] [PubMed]
- Macdonald-Clarke, C.J.; Martin, B.R.; McCabe, L.D.; McCabe, G.P.; Lachcik, P.J.; Wastney, M.; Weaver, C.M. Bioavailability of potassium from potatoes and potassium gluconate: A randomized dose response trial. Am. J. Clin. Nutr. 2016, 104, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, K.; Kido, Y.; Akaishi, Y.; Quitan, A.; Kida, T. Synthesis of Cu2O/CuO Nanocrystals and Their Application to H2S Sensing. Sensors 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.C.; von Dreele, R.B. General structure analysis system. Los Alamos Natl. Lab. Rep. LAUR 1994, 96, 86. [Google Scholar]
- Foo, M.L.; Huang, Q.; Lynn, J.W.; Lee, W.-L.; Klimczuk, T.; Hagemann, I.S.; Ong, N.P.; Cava, R.J. Synthesis, structure and physical properties of Ru ferrites: BaMRu5O11 (M=Li and Cu) and BaM′2Ru4O11 (M′=Mn, Fe and Co). J. Solid State Chem. 2006, 179, 563–572. [Google Scholar] [CrossRef]
- Howard, C.J. The approximation of asymmetric neutron powder diffraction peaks by sums of Gaussians. J. Appl. Cryst. 1982, 15, 615–620. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye-Scherrer synchrotron X-ray data from Al2O3. J. Appl. Cryst. 1987, 20, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mei, Z.; Liu, L.; Liang, H.; Azarov, A.; Kuznetsov, A.; Liu, Y.; Ji, A.; Meng, Q.; Du, X. Probing Defects in Nitrogen-Doped Cu2O. Sci. Rep. 2014, 4, 7240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Zhu, C.; Osherov, A.; Panzer, M.J. Surface chemistry of electrodeposited Cu2O films studied by XPS. Electrochem. Acta 2013, 111, 771–778. [Google Scholar] [CrossRef]
- Aria, A.I.; Kidambi, P.R.; Weatherup, R.S.; Xiao, L.; Williams, J.A.; Hofmann, S. Time Evolution of the Wettability of Supported Graphene under Ambient Air Exposure. J. Phys. Chem. C 2016, 120, 2215–2224. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Liu, G. Chapter Ten—Facet Control of Photocatalysts for Water Splitting. Semicond. Semimet. 2017, 97, 349–391. [Google Scholar]
- He, P.; Shen, X.; Gao, H. Size-controlled preparation of Cu2O octahedron nanocrystals and studies on their optical absorption. J. Colloid Interface Sci. 2005, 284, 510–515. [Google Scholar] [CrossRef]
- Poulopoulos, P.; Baskoutas, S.; Pappas, S.D.; Garoufalis, C.S.; Droulias, S.A.; Zamani, A.; Kapaklis, V. Intense Quantum Confinement Effects in Cu2O Thin Films. J. Phys. Chem. C 2011, 115, 14839–14843. [Google Scholar] [CrossRef]
- Niveditha, C.V.; Fatima, M.J.J.; Sindhu, S. Comprehensive Interfacial Study of Potentio-Dynamically Synthesized Copper Oxide Thin Films for Photoelectrochemical Applications. J. Electrochem. Soc. 2016, 163, 426–433. [Google Scholar] [CrossRef]
- Hubička, Z.; Zlámal, M.; Čada, M.; Kment, Š.; Krýsa, J. Photo-electrochemical stability of copper oxide photocathodes deposited by reactive high power impulse magnetron sputtering. Catal. Today 2019, 328, 29–34. [Google Scholar] [CrossRef]
- Dunn, H.K.; Feckl, J.M.; Müller, A.; Fattakhova-Rohlfing, D.; Morehead, S.G.; Roos, J.; Peter, L.M.; Scheu, C.; Bein, T. Tin doping speeds up hole transfer during light-driven water oxidation at hematite photoanodes. Phys. Chem. Chem. Phys. 2014, 16, 24610–24620. [Google Scholar] [CrossRef] [Green Version]
- Kecsenovity, E.; Endrődi, B.; Tóth, P.S.; Zou, Y.; Dryfe, R.A.W.; Rajeshwar, K.; Janáky, C. Enhanced Photoelectrochemical Performance of Cuprous Oxide/Graphene Nanohybrids. J. Am. Chem. Soc. 2017, 139, 6682–6692. [Google Scholar] [CrossRef] [PubMed]







| Parameter | 4.5 V-3H | 4.5 V-1H | 5.0 V | 6.0 V | 7.0 V | 8.0 V |
|---|---|---|---|---|---|---|
| χ2 | 1.17 | 1.32 | 1.31 | 1.33 | 1.42 | 1.29 |
| Rwp (%) | 10.84 | 10.03 | 9.94 | 10.46 | 10.14 | 10.42 |
| Rp (%) | 8.51 | 7.95 | 7.79 | 8.01 | 7.93 | 8.07 |
| a (Å) | 4.26 | 4.26 | 4.27 | 4.26 | 4.26 | 4.26 |
| Cell Vol (Å3) | 77.53 | 77.53 | 77.69 | 77.36 | 77.42 | 77.43 |
| Cu-O bond length (Å) | 1.85 | 1.85 | 1.85 | 1.84 | 1.84 | 1.84 |
| Cu-O-Cu bond angle (˚) | 109.47 | 109.47 | 109.47 | 109.47 | 109.47 | 109.47 |
| Crystallite size (nm) | 27.1 | 21.7 | 20.3 | 16.2 | 15.6 | 14.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McWilliams, S.; Flynn, C.D.; McWilliams, J.; Arnold, D.C.; Wahyuono, R.A.; Undisz, A.; Rettenmayr, M.; Ignaszak, A. Nanostructured Cu2O Synthesized via Bipolar Electrochemistry. Nanomaterials 2019, 9, 1781. https://doi.org/10.3390/nano9121781
McWilliams S, Flynn CD, McWilliams J, Arnold DC, Wahyuono RA, Undisz A, Rettenmayr M, Ignaszak A. Nanostructured Cu2O Synthesized via Bipolar Electrochemistry. Nanomaterials. 2019; 9(12):1781. https://doi.org/10.3390/nano9121781
Chicago/Turabian StyleMcWilliams, Steven, Connor D. Flynn, Jennifer McWilliams, Donna C. Arnold, Ruri Agung Wahyuono, Andreas Undisz, Markus Rettenmayr, and Anna Ignaszak. 2019. "Nanostructured Cu2O Synthesized via Bipolar Electrochemistry" Nanomaterials 9, no. 12: 1781. https://doi.org/10.3390/nano9121781