Effects of Palladium Precursors on the Activity of Palladium Nanocatalysts for the Oxidation of Volatile Organic Components
Abstract
:1. Introduction
2. Experiment
2.1. Materials
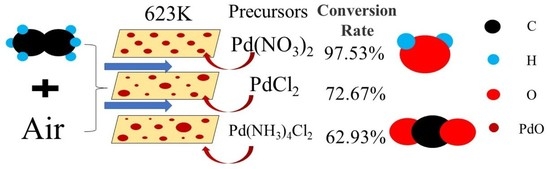
2.2. Preparation of Catalysts
2.3. Evaluation of Catalytic Oxidation Activities
2.4. Characterization of Catalysts
3. Results and Discussion
3.1. Analysis of Catalysts Composition
3.2. Structural Characterization
3.3. Characterization of Metal Dispersion
3.4. Reductive Performance Characterization
3.5. Surface Acidity and Valence Analysis
3.6. Mechanism and Products
3.7. Catalytic Oxidation Performance
3.8. Water Resistance and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, A.; Yin, S.; Yuan, M.; Xu, Y.; Yu, S.; Zhang, D.; Lu, X.; Zhang, R. Characteristics, source analysis and chemical reactivity of ambient VOCs in a heavily polluted city of central China. Atmos. Pollut. Res. 2022, 13, 101390. [Google Scholar] [CrossRef]
- Bedia, C.; Cardoso, P.; Dalmau, N.; Garreta-Lara, E.; Gómez-Canela, C.; Gorrochategui, E.; Navarro-Reig, M.; Ortiz-Villanueva, E.; Puig-Castellví, F.; Tauler, R. Chapter Nineteen—Applications of Metabolomics Analysis in Environmental Research. In Comprehensive Analytical Chemistry; Jaumot, J., Bedia, C., Tauler, R., Eds.; Elsevier: Amsterdam, Netherlands, 2018; Volume 82, pp. 533–582. [Google Scholar]
- Li, M.; Zhang, Q.; Zheng, B.; Tong, D.; Lei, Y.; Liu, F.; Hong, C.; Kang, S.; Yan, L.; Zhang, Y.; et al. Persistent growth of anthropogenic non-methane volatile organic compound (NMVOC) emissions in China during 1990–2017: Drivers, speciation and ozone formation potential. Atmos. Chem. Phys. 2019, 19, 8897–8913. [Google Scholar] [CrossRef] [Green Version]
- Barakat, T.; Idakiev, V.; Cousin, R.; Shao, G.S.; Yuan, Z.Y.; Tabakova, T.; Siffert, S. Total oxidation of toluene over noble metal based Ce, Fe and Ni doped titanium oxides. Appl. Catal. B 2014, 146, 138–146. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B 2010, 98, 180–185. [Google Scholar] [CrossRef]
- He, C.; Li, J.; Li, P.; Cheng, J.; Hao, Z.; Xu, Z.-P. Comprehensive investigation of Pd/ZSM-5/MCM-48 composite catalysts with enhanced activity and stability for benzene oxidation. Appl. Catal. B 2010, 96, 466–475. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Fang, D.; Han, J.; Dang, F.; Yang, M. Study of the use of a Pd–Pt-based catalyst for the catalytic combustion of storage tank VOCs. Int. J. Hydrogen Energy 2020, 45, 22732–22743. [Google Scholar] [CrossRef]
- Peng, H.; Dong, T.; Yang, S.; Chen, H.; Yang, Z.; Liu, W.; He, C.; Wu, P.; Tian, J.; Peng, Y.; et al. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes. Nat. Commun. 2022, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wiebenga, M.H.; Carrillo, C.; Gaudet, J.R.; Pham, H.N.; Kunwar, D.; Oh, S.H.; Qi, G.; Kim, C.H.; Datye, A.K. Design considerations for low-temperature hydrocarbon oxidation reactions on Pd based catalysts. Appl. Catal. B 2018, 236, 436–444. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, Y.X.; Wu, N.Z.; Xie, Y.C.; Murwani, I.; Kemnitz, E. Total oxidation of methane at low temperature over Pd/TiO2/Al2O3: Effects of the support and residual chlorine ions. Appl. Catal. B 2004, 50, 59–66. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.; He, H. Complete oxidation of o-xylene over Pd/Al2O3 catalyst at low temperature. Catal. Today 2008, 139, 15–23. [Google Scholar] [CrossRef]
- Cui, W.; Li, S.; Wang, D.; Deng, Y.; Chen, Y. High reactivity and sintering resistance of CH4 oxidation over modified Pd/Al2O3. Catal. Commun. 2019, 119, 86–90. [Google Scholar] [CrossRef]
- Dong, T.; Liu, W.; Ma, M.; Peng, H.; Yang, S.; Tao, J.; He, C.; Wang, L.; Wu, P.; An, T. Hierarchical zeolite enveloping Pd-CeO2 nanowires: An efficient adsorption/catalysis bifunctional catalyst for low temperature propane total degradation. Chem. Eng. J. 2020, 393, 124717. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Yang, M.; Yang, H.; Liu, M.; Dang, F. Pt modulates the electronic structure of Pd to improve the performance of Pd-based catalytic combustion catalyst. Int. J. Hydrogen Energy 2021, 46, 18391–18400. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, J.; Chi, X.; Wu, K.; Song, L.; Li, T.; Zhao, Y.; Huang, H.; Chen, P.; Wu, J.; et al. Pd-Promoted Co2NiO4 with lattice CoONi and interfacial PdO activation for highly efficient methane oxidation. Appl. Catal. B 2021, 292, 120201. [Google Scholar] [CrossRef]
- Chen, S.; Yao, Y.; Lan, L.; Cao, Y.; Yan, C.; Gong, M.; Chen, Y. Effect of Zirconium Precursor on Performance of Pd/Ce0.45Zr0.45La0.1O1.95 Three-Way Catalyst. Chin. J. Catal. 2012, 33, 1762–1771. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, Y.; Liu, J.; Xu, X.; Xu, J.; Fang, X.; Wang, X. Remarkable Pd/SnO2 nano-rod catalysts with ultra-low Pd content for toluene combustion: Clarifying the effect of SnO2 morphology on the valence states of the supported Pd species and the vital role of Pd0. Appl. Catal. A 2022, 636, 118576. [Google Scholar] [CrossRef]
- Sanz, O.; Delgado, J.J.; Navarro, P.; Arzamendi, G.; Gandía, L.M.; Montes, M. VOCs combustion catalysed by platinum supported on manganese octahedral molecular sieves. Appl. Catal. B 2011, 110, 231–237. [Google Scholar] [CrossRef]
- Xie, K.; Wang, Z.; Jiang, B.; Zhao, B.; Zuo, S. Synthesis of quantitative sulfur-poisoned Pd/γ-Al2O3 and its deactivation mechanism for catalytic combustion of toluene. Appl. Catal. A 2022, 639, 118641. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wang, J. DFT study on the regeneration of Pt/γ-Al2O3 catalyst: The effect of chlorine on the redispersion of metal species. Appl. Surf. Sci. 2021, 545, 148988. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Yaseen, M.; Naeem, M.; Khan, A. Confinement of Au, Pd and Pt nanoparticle with reduced sizes: Significant improvement of dispersion degree and catalytic activity. Microporous Mesoporous Mater. 2022, 337, 111927. [Google Scholar] [CrossRef]
- Xiong, J.; Mo, S.; Song, L.; Fu, M.; Chen, P.; Wu, J.; Chen, L.; Ye, D. Outstanding stability and highly efficient methane oxidation performance of palladium-embedded ultrathin mesoporous Co2MnO4 spinel catalyst. Appl. Catal. A 2020, 598, 117571. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Lu, E.; Wang, Z.; Gong, X.; Yu, Z.; Guo, Y.; Wang, L.; Guo, Y.; Zhan, W.; et al. Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 2019, 10, 1611. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, C.; Sun, H.-L.; Fu, G.; Chen, S.; Zhang, Y.-J.; Chen, B.-H.; Anema, J.R.; Yang, Z.-L.; Li, J.-F.; et al. In situ dynamic tracking of heterogeneous nanocatalytic processes by shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 15447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, G.; Zheng, P.; Dang, F.; Han, J. Carbon deposits during catalytic combustion of toluene on Pd–Pt-based catalysts. Catal. Sci. Technol. 2020, 10, 2452–2461. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, S.; Wang, T.; Xing, D.; Yue, X.; Wang, M.; Wang, S. Insights into the promotion mechanism of ceria-zirconia solid solution to ethane combustion over Pt-based catalysts. J. Catal. 2022, 405, 129–139. [Google Scholar] [CrossRef]
- Liang, X.; Qi, F.; Liu, P.; Wei, G.; Su, X.; Ma, L.; He, H.; Lin, X.; Xi, Y.; Zhu, J.; et al. Performance of Ti-pillared montmorillonite supported Fe catalysts for toluene oxidation: The effect of Fe on catalytic activity. Appl. Clay Sci. 2016, 132–133, 96–104. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, W.; Wang, X.; Lu, G. Sandwich-structured CeO2@ZSM-5 hybrid composites for catalytic oxidation of 1, 2-dichloroethane: An integrated solution to coking and chlorine poisoning deactivation. Appl. Catal. B 2017, 203, 31–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Cordi, E.M.; Falconer, J.L. Oxidation of Volatile Organic Compounds on Al2O3, Pd/Al2O3, and PdO/Al2O3 Catalysts. J. Catal. 1996, 162, 104–117. [Google Scholar] [CrossRef]
- Solsona, B.E.; Garcia, T.; Jones, C.; Taylor, S.H.; Carley, A.F.; Hutchings, G.J. Supported gold catalysts for the total oxidation of alkanes and carbon monoxide. Appl. Catal. A 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Nakamura, T.; Ohshima, M.-a.; Kurokawa, H.; Miura, H. Effects of Removing Residual Chlorine on the Hydrogenation of Aromatic Hydrocarbons over Supported Ru Catalysts. Chem. Lett. 2009, 39, 62–63. [Google Scholar] [CrossRef]












| Catalyst | XRF (Pd) | XRF (Cl) | ICP-OES (Pd2+) |
|---|---|---|---|
| Pd-1 | 0.189% w/w | - | 0.184% w/w |
| Pd-2 | 0.198% w/w | 0.099% w/w | 0.193% w/w |
| Pd-3 | 0.186% w/w | 0.087% w/w | 0.183% w/w |
| Catalyst | SBET (m2/g) | VBJH (cm3/g) | DBJH (nm) |
|---|---|---|---|
| Al2O3/COR | 16.5 | 0.0372 | 9.09 |
| Pd-1 | 12.2 | 0.0298 | 8.63 |
| Pd-2 | 11.9 | 0.0293 | 9.03 |
| Pd-3 | 12.6 | 0.0299 | 8.64 |
| γ-Al2O3 | 140 | 0.482 | 12.3 |
| Catalyst | Metal Dispersion |
|---|---|
| Pd-1 | 17.7% |
| Pd-2 | 2.68% |
| Pd-3 | 4.38% |
| Catalyst | Weak Acid Sites (mmol/g) | Moderate Strong Acid Sites (mmol/g) | Strong Acid Sites (mmol/g) |
|---|---|---|---|
| Pd-1 | 0.0818 | 0.0061 | 0.0090 |
| Pd-2 | 0.0737 | 0.0177 | 0.0078 |
| Pd-3 | 0.0743 | 0.0093 | 0.0049 |
| Al2O3/COR | 0.0533 | 0.0076 | 0.0035 |
| Catalyst | Pd2+/(Pd2+ + Pd4+) |
|---|---|
| Pd-1 | 70.86% |
| Pd-2 | 59.38% |
| Pd-3 | 63.68% |
| Catalysts | Toluene (ppm) | GHSV | T50 (K) | T90 (K) | Ref. |
|---|---|---|---|---|---|
| Au/CoOX | 5000 | 15,000 h−1 | - | 523 | [31] |
| Pt/CZ11 | 1000 | 50 L g−1 h−1 | 543 | 581 | [26] |
| Pd-1 | 2000 | 10,000 h−1 | 556 | 574 | This paper |
| Catalyst | XRF (Cl) |
|---|---|
| Pd-2 | 0.099% w/w |
| Pd-3 | 0.087% w/w |
| Pd-2-Cl | 0.015% w/w |
| Pd-3-Cl | 0.014% w/w |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Cai, Q.; Li, X.; Han, E.; Sun, Y.; Lu, Y.; Cai, Z.; Yu, H. Effects of Palladium Precursors on the Activity of Palladium Nanocatalysts for the Oxidation of Volatile Organic Components. Nanomaterials 2023, 13, 1189. https://doi.org/10.3390/nano13071189
Li Q, Cai Q, Li X, Han E, Sun Y, Lu Y, Cai Z, Yu H. Effects of Palladium Precursors on the Activity of Palladium Nanocatalysts for the Oxidation of Volatile Organic Components. Nanomaterials. 2023; 13(7):1189. https://doi.org/10.3390/nano13071189
Chicago/Turabian StyleLi, Qingtao, Qi Cai, Xiaoyun Li, Enshan Han, Yanmin Sun, Yanfei Lu, Zhe Cai, and Haibin Yu. 2023. "Effects of Palladium Precursors on the Activity of Palladium Nanocatalysts for the Oxidation of Volatile Organic Components" Nanomaterials 13, no. 7: 1189. https://doi.org/10.3390/nano13071189