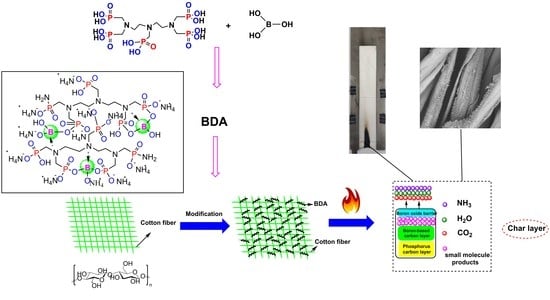
Reactive Flame-Retardant Cotton Fabric Coating: Combustion Behavior, Durability, and Enhanced Retardant Mechanism with Ion Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BDA
2.3. Flame-Retardant Treatment of Cotton Fabrics
2.4. Characterization
3. Results and Discussion
3.1. Finishing Conditions
3.2. FTIR Spectra Analysis
3.3. XPS Analysis
3.4. Flame Retardant Properties and Durability
3.5. Thermal Stability
3.6. TG-IR Analysis
3.7. Surface Morphology
3.8. Flame Retardant Mechanism
3.9. Tensile Strength and Whiteness of Cotton Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Nazir, R.; Gaan, S. Recent developments in P(O/S)-N containing flame retardants. J. Appl. Polym. Sci. 2020, 137, 47910. [Google Scholar] [CrossRef] [Green Version]
- Bolanos, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef]
- He, S.H.; Liu, C.; Chi, X.W.; Zhang, Y.D.; Yu, G.; Wang, H.S.; Li, B.; Peng, H. Bio-inspired lightweight pulp foams with improved mechanical property and flame retardancy via borate cross-linking. Chem. Eng. J. (Lausanne) 2019, 371, 34–42. [Google Scholar] [CrossRef]
- Intharapat, P.; Nakason, C.; Kongnoo, A. Preparation of boric acid supported natural rubber as a reactive flame retardant and its properties. Polym. Degrad. Stab. 2016, 128, 217–227. [Google Scholar] [CrossRef]
- Wang, S.J.; Bian, C.; Jia, B.B.; Wang, Y.N.; Jing, X.L. Structure and thermal pyrolysis mechanism of poly(resorcinol borate) with high char yield. Polym. Degrad. Stab. 2016, 130, 328–337. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Dong, Y.P. Synergistic flame-retardant effect and mechanisms of boron/phosphorus compounds on epoxy resins. Polym. Adv. Technol. 2018, 29, 641–648. [Google Scholar] [CrossRef]
- Davis, R.; Li, Y.C.; Gervasio, M.; Luu, J.; Kim, Y.S. One-Pot, Bioinspired Coatings To Reduce the Flammability of Flexible Polyurethane Foams. ACS Appl. Mater. Interfaces 2015, 7, 6082–6092. [Google Scholar] [CrossRef]
- Lai, X.J.; Zeng, X.R.; Li, H.Q.; Zhang, H.L. Effect of Polyborosiloxane on the Flame Retardancy and Thermal Degradation of Intumescent Flame Retardant Polypropylene. J. Macromol. Sci. Part B Phys. 2014, 53, 721–734. [Google Scholar] [CrossRef]
- Chen, Y.S.; Duan, H.J.; Ji, S.; Ma, H.R. Novel phosphorus/nitrogen/boron-containing carboxylic acid as co-curing agent for fire safety of epoxy resin with enhanced mechanical properties. J. Hazard. Mater. 2021, 402, 123769. [Google Scholar] [CrossRef]
- Xu, M.J.; Ma, K.; Liu, C.; Li, B. Synthesis of the poly(phosphoric-boric acid) piperazine and its application as an effective flame retardant for epoxy resins. Polym. Eng. Sci. 2018, 58, 1858–1867. [Google Scholar] [CrossRef]
- Zhu, W.J.; Hao, S.S.; Yang, M.Y.; Cheng, B.W.; Zhang, J.M. A synergistic flame retardant of glycosyl cross-linking boron acid and ammonium salt of phytic acid to enhance durable flame retardancy of cotton fabrics. Cellulose 2020, 27, 9699–9710. [Google Scholar] [CrossRef]
- Zhu, W.J.; Yang, M.Y.; Huang, H.; Dai, Z.; Cheng, B.W.; Hao, S.S. A phytic acid-based chelating coordination embedding structure of phosphorus-boron-nitride synergistic flame retardant to enhance durability and flame retardancy of cotton. Cellulose 2020, 27, 4817–4829. [Google Scholar] [CrossRef]
- Xia, L.; Liu, J.; Li, Z.; Wang, X.B.; Wang, P.; Wang, D.; Hu, X.H. Synthesis and flame retardant properties of new boron-containing polyurethane. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 560–568. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.L.; Li, M.M.; Zhu, J.H.; Ge, A.X.; Liu, L.F.; Yu, J.Y. Cellulose-based composite thermal-insulating foams toward eco-friendly, flexible and flame-retardant. Carbohydr. Polym. 2021, 273, 118544. [Google Scholar] [CrossRef]
- Wuorimaa, A.; Jokela, R.; Aksela, R. Recent developments in the stabilization of hydrogen peroxide bleaching of pulps: An overview. Nord. Pulp Pap. Res. J. 2006, 21, 435–443. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Peacock, C.L.; Benning, L.G. Investigating the Effectiveness of Phosphonate Additives in Hindering the Calcium Sulfate Dihydrate Scale Formation. Ind. Eng. Chem. Res. 2020, 59, 14970–14980. [Google Scholar] [CrossRef]
- Zeino, A.; Albakri, M.; Khaled, M.; Zarzour, M. Comparative study of the synergistic effect of ATMP and DTPMPA on CaSO4 scale inhibition and evaluation of induction time effect. J. Water Process Eng. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Studnik, H.; Liebsch, S.; Forlani, G.; Wieczorek, D.; Kafarski, P.; Lipok, J. Amino polyphosphonates-chemical features and practical uses, environmental durability and biodegradation. New Biotechnol. 2015, 32, 1–6. [Google Scholar] [CrossRef]
- Chen, X.X.; Wang, H.Y.; Wang, C.J.; Zhang, W.B.; Lv, C.J.; Zhu, Y.J. A novel antiscaling and anti-corrosive polymer-based functional coating. J. Taiwan Inst. Chem. Eng. 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Jaworska, J.; Van Genderen-Takken, H.; Hanstveit, A.; van de Plassehe, E.; Feijtel, T. Environmental risk assessment of phosphonates, used in domestic laundry and cleaning agents in the Netherlands. Chemosphere 2002, 47, 655–665. [Google Scholar] [CrossRef]
- Cigala, R.M.; Cordaro, M.; Crea, F.; De Stefano, C.; Fracassetti, V.; Marchesi, M.; Milea, D.; Sammartano, S. Acid-Base Properties and Alkali and Alkaline Earth Metal Complex Formation in Aqueous Solution of Diethylenetriamine-N,N,N ′,N ″,N ″-pentakis(methylenephosphonic acid) Obtained by an Efficient Synthetic Procedure. Ind. Eng. Chem. Res. 2014, 53, 9544–9553. [Google Scholar] [CrossRef]
- Jin, X.D.; Gu, X.Y.; Chen, C.; Tang, W.F.; Li, H.F.; Liu, X.D.; Bourbigot, S.; Zhang, Z.W.; Sun, J.; Zhang, S. The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 12235–12250. [Google Scholar] [CrossRef]
- Zheng, D.D.; Zhou, J.F.; Wang, Y.; Zhang, F.X.; Zhang, G.X. A reactive flame retardant ammonium salt of diethylenetriaminepenta(methylene-phosphonic acid) for enhancing flame retardancy of cotton fabrics. Cellulose 2018, 25, 787–797. [Google Scholar] [CrossRef]
- Zheng, D.D.; Zhou, J.F.; Zhong, L.; Zhang, F.X.; Zhang, G.X. A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 2016, 23, 2211–2220. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Oreshonkov, A.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V.; Razumkova, I.A.; Atuchin, V.V. Crystal Structure, Vibrational, Spectroscopic and Thermochemical Properties of Double Sulfate Crystalline Hydrate [CsEu(H2O)3(SO4)2]. H2O and Its Thermal Dehydration Product CsEu(SO4)2. Crystals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Shang, K.; Liao, W.; Wang, Y.Z. Thermally stable and flame-retardant poly(vinyl alcohol)/montmorillonite aerogel via a facile heat treatment. Chinese Chem. Lett. 2018, 29, 433–436. [Google Scholar] [CrossRef]
- Maleki, M.; Beitollahi, A.; Javadpour, J.; Yahya, N. Dual template route for the synthesis of hierarchical porous boron nitride. Ceram. Int. 2015, 41, 3806–3813. [Google Scholar] [CrossRef]
- Liu, L.; Fu, M.T.; Wang, Z.Z. Synthesis of Boron-Containing Toughening Agents and Their Application in Phenolic Foams. Ind. Eng. Chem. Res. 2015, 54, 1962–1970. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeey, M.S.; Lesnikov, M.K.; Sterkhova, I.V.; Atuchin, V.V. Thiobarbiturate and barbiturate salts of pefloxacin drug: Growth, structure, thermal stability and IR-spectra. J. Mol. Struct. 2017, 1149, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Xu, M.J.; Wang, Q.; Li, B. A novel durable flame retardant cotton fabric produced by surface chemical grafting of phosphorus- and nitrogen-containing compounds. Cellulose 2017, 24, 4069–4081. [Google Scholar] [CrossRef]
- Chan, S.Y.; Si, L.P.; Lee, K.I.; Ng, P.F.; Chen, L.; Yu, B.; Hu, Y.; Yuen, R.K.K.; Xin, J.H.; Fei, B. A novel boron-nitrogen intumescent flame retardant coating on cotton with improved washing durability. Cellulose 2018, 25, 843–857. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.S.; Wang, X.H. Influence of nano-silica on the flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V.; Sidorenko, M.Y.; Dmitrushkov, M.S. Crystal structure and properties of the precursor [Ni(H2O)6(HTBA)2.2H2O and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe). Polyhedron 2014, 70, 71–76. [Google Scholar] [CrossRef]
- Vasiljevic, J.; Gorjanc, M.; Tomsic, B.; Orel, B.; Jerman, I.; Mozetic, M.; Vesel, A.; Simoncic, B. The surface modification of cellulose fibres to create super-hydrophobic, oleophobic and self-cleaning properties. Cellulose 2013, 20, 277–289. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, D.C.; Zhang, Y.; Cai, W.; Yao, C.X.; Hu, Y.; Hu, W.Z. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2019, 362, 482–494. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Pervukhina, N.V. Electronic and structural parameters of phosphorus-oxygen bonds in inorganic phosphate crystals. Surf. Rev. Lett. 2008, 15, 391–399. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Meng, G.S.; Lin, Z.S. The electronic structure of RbTiOPO4 and the effects of the A-site cation substitution in KTiOPO4-family crystals. J. Phys. Condens. Matter 2012, 24, 405503. [Google Scholar] [CrossRef]
- Caron, T.; Palmas, P.; Frenois, C.; Methivier, C.; Pasquinet, E.; Pradier, C.M.; Serein-Spirau, F.; Hairault, L.; Montmeat, P. Detection of hydrogen peroxide using dioxazaborocanes: Elucidation of the sensing mechanism at the molecular level by NMR and XPS measurements. New J. Chem. 2020, 44, 4114–4121. [Google Scholar] [CrossRef]
- Ding, S.L.; Zheng, S.J.; Xie, M.J.; Peng, L.M.; Guo, X.F.; Ding, W.P. One-pot synthesis of boron-doped mesoporous carbon with boric acid as a multifunction reagent. Microporous Mesoporous Mater. 2011, 142, 609–613. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Kokh, A.E.; Pokrovsky, L.D. X-ray photoelectron spectroscopy study of β-BaB2O4 optical surface. Appl. Surf. Sci. 2004, 223, 352–360. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Adichtchev, S.V.; Bazarov, B.G.; Bazarova, Z.G.; Gavrilova, T.A.; Grossman, V.G.; Kesler, V.G.; Meng, G.S.; Lin, Z.S.; Surovtsev, N.V. Electronic structure and vibrational properties of KRbAl2B2O7. Mater. Res. Bull. 2013, 48, 929–934. [Google Scholar] [CrossRef]
- Panayiotatos, Y.; Logothetidis, S.; Handrea, M.; Kautek, W. Homogeneous and amorphous sputtered Sp(3)-bonded BN films at RT: A stress, spectroscopic ellipsometry and XPS study. Diamond Relat. Mater. 2003, 12, 1151–1156. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Botteri, L.; Alongi, J.; Malucelli, G. Synergistic effects occurring between water glasses and urea/ammonium dihydrogen phosphate pair for enhancing the flame retardancy of cotton. Cellulose 2015, 22, 2825–2835. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V. Synthesis and thermal transformation of a neodymium(III) complex [Nd(HTBA)2(C2H3O2)(H2O)2].2H2O to non-centrosymmetric oxosulfate Nd2O2SO4. J. Coord. Chem. 2015, 68, 1865–1877. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Solovyov, L.A.; Lesnikov, M.K.; Vereshchagin, S.N.; Atuchin, V.V. Hydrated and anhydrous cobalt (II) barbiturates: Crystal structures, spectroscopic and thermal properties. Inorg. Chim. Acta 2017, 467, 39–45. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Samoilo, A.S.; Atuchin, V.V. Influence of alkyl substituents in 1,3-diethyl-2-thiobarbituric acid on the coordination environment in M(H2O)2(1,3-diethyl-2-thiobarbiturate)2 M = Ca2+, Sr2+. J. Coord. Chem. 2016, 69, 957–965. [Google Scholar] [CrossRef]
- Miao, Z.W.; Yan, D.P.; Wang, X.D.; Zhang, X.F.; Zhou, W.Q.; Qiu, M.N.; Yang, F.; Wu, Z.P. New flame retardant epoxy resins based on cyclophosphazene-derived curing agents. Chinese Chem. Lett. 2021, 33, 4026–4032. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Chang, S.C.; Condon, B.; Thomas, T.P.; Azadi, P. Thermal decomposition reactions of cotton fabric treated with piperazine-phosphonates derivatives as a flame retardant. J. Anal. Appl. Pyrolysis. 2014, 110, 122–129. [Google Scholar] [CrossRef]
- Rosace, G.; Castellano, A.; Trovato, V.; Iacono, G.; Malucelli, G. Thermal and flame retardant behaviour of cotton fabrics treated with a novel nitrogen-containing carboxyl-functionalized organophosphorus system. Carbohydr. Polym. 2018, 196, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Xia, Y.Z.; Shi, M.W.; Yan, X. The flame retardancy of alginate/flame retardant viscose fibers investigated by vertical burning test and cone calorimeter. Chinese Chem. Lett. 2018, 29, 489–492. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, L.; Zhang, C.; Wang, P.; Zhang, F.X.; Zhang, G.X. Novel High-Efficiency Casein-Based P-N-Containing Flame Retardants with Multiple Reactive Groups for Cotton Fabrics. ACS Sustainable Chem. Eng. 2019, 7, 13999–14008. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Wang, S.; Jing, X. Cross-linked polymers based on B–O bonds: Synthesis, structure and properties. Mater. Chem. Front. 2021, 5, 5534–5548. [Google Scholar] [CrossRef]
- Deng, P.; Shi, Y.; Liu, Y.S.; Liu, Y.; Wang, Q. Solidifying process and flame retardancy of epoxy resin cured with boron-containing phenolic resin. Appl. Surf. Sci. 2018, 427, 894–904. [Google Scholar] [CrossRef]
- Wang, D.F.; Zhong, L.; Zhang, C.; Zhang, F.X.; Zhang, G.X. A novel reactive phosphorous flame retardant for cotton fabrics with durable flame retardancy and high whiteness due to self-buffering. Cellulose 2018, 25, 5479–5497. [Google Scholar] [CrossRef]











| Sample | XPS Atomic Percent (at.%) | ||||
|---|---|---|---|---|---|
| C | O | N | B | P | |
| Control sample | 73.45 | 21.11 | - | - | - |
| BDA-90 treated sample | 66.15 | 22.01 | 4.72 | 2.67 | 1.75 |
| BDA-90 after burning | 70.88 | 14.28 | 6.61 | 3.93 | 3.23 |
| Sample | TTI (s) | HRR (MJ/m2) | pkHRR (W/m2) | THR (MJ/m2) | EHC (MJ/kg) | TSR (m2/m2) | CO2/CO (kg/kg) | Residue (%) |
|---|---|---|---|---|---|---|---|---|
| Control sample (CV %) | 9 | 46.44 (10.30) | 206.2 (9.48) | 7.76 (3.97) | 19.3 (28.66) | 113.0 (14.51) | 41.41 (12.31) | - |
| BDA-90 (CV %) | 9 | 15.84 (8.57) | 43.50 (17.16) | 3.84 (6.67) | 10.2 (23.36) | 67.3 (23.47) | 3.41 (6.01) | 20.1 (6.26) |
| Laundering Cycles | LOI (%) | |||
|---|---|---|---|---|
| BDA-30 | BDA-60 | BDA-90 | BDA-120 | |
| 1 | 32.0 | 33.6 | 36.1 | 33.7 |
| 10 | 31.8 | 32.3 | 32.9 | 32.5 |
| 30 | 31.0 | 31.7 | 32.6 | 32.2 |
| 50 | 27.8 | 28.9 | 30.3 | 30.1 |
| Sample | Ta Onset (°C) | Ta Max (°C) | Ra Max (%/°C) | Tb Onset (°C) | Tb Max (°C) | Rb Max (%/°C) | Residue (%) |
|---|---|---|---|---|---|---|---|
| Control sample | 71.6 | 99.0 | 0.0161 | 307.7 | 343.2 | 2.0414 | 19.57 |
| BDA-30 | 67.3 | 88.4 | 0.0550 | 267.4 | 285.8 | 2.3141 | 36.21 |
| BDA-60 | 66.6 | 88.3 | 0.0516 | 269.1 | 285.3 | 2.7191 | 34.95 |
| BDA-90 | 68.5 | 90.8 | 0.0556 | 270.6 | 287.4 | 2.4666 | 36.33 |
| BDA-120 | 67.3 | 89.2 | 0.0516 | 268.5 | 285.1 | 2.6325 | 34.84 |
| BDA | 103.7 | 147.6 | 0.8335 | 252.6 | 260.0 | 1.9297 | 42.26 |
| Chemical bond | P–C | C–N | P–N | C–O | C–C |
| Bond energy(kJ/mol) | 264 | 293 | 300 | 326 | 335 |
| Chemical bond | B–C | B–N | P–O | P=O | B–O |
| Bond energy(kJ/mol) | 356 | 389 | 410 | 585 | 774 |
| Sample | Tensile Strengths (N) | The Loss Rate of Breaking Strength (%) |
|---|---|---|
| Control sample | 530 | - |
| BDA-30 | 472 | 10.94 |
| BDA-60 | 455 | 14.15 |
| BDA-90 | 409 | 22.83 |
| BDA-120 | 393 | 25.85 |
| LCs | Whiteness Value (Whiteness Ratio) | |||
|---|---|---|---|---|
| BDA-30 | BDA-60 | BDA-90 | BDA-120 | |
| 1 | 64.68 (85.4) | 63.94 (84.4) | 61.84 (81.7) | 64.74 (85.5) |
| 10 | 66.54 (87.9) | 65.20 (86.1) | 63.85 (84.3) | 65.84 (86.9) |
| 30 | 67.88 (89.6) | 67.04 (88.5) | 64.54 (85.2) | 66.90 (88.3) |
| 50 | 69.40 (91.6) | 67.98 (89.8) | 66.24 (87.5) | 67.60 (89.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Wang, Q.; Yang, M.; Li, M.; Zheng, C.; Li, D.; Zhang, X.; Cheng, B.; Dai, Z. Reactive Flame-Retardant Cotton Fabric Coating: Combustion Behavior, Durability, and Enhanced Retardant Mechanism with Ion Transfer. Nanomaterials 2022, 12, 4048. https://doi.org/10.3390/nano12224048
Zhu W, Wang Q, Yang M, Li M, Zheng C, Li D, Zhang X, Cheng B, Dai Z. Reactive Flame-Retardant Cotton Fabric Coating: Combustion Behavior, Durability, and Enhanced Retardant Mechanism with Ion Transfer. Nanomaterials. 2022; 12(22):4048. https://doi.org/10.3390/nano12224048
Chicago/Turabian StyleZhu, Wenju, Qing Wang, Mingyang Yang, Minjing Li, Chunming Zheng, Dongxiang Li, Xiaohan Zhang, Bowen Cheng, and Zhao Dai. 2022. "Reactive Flame-Retardant Cotton Fabric Coating: Combustion Behavior, Durability, and Enhanced Retardant Mechanism with Ion Transfer" Nanomaterials 12, no. 22: 4048. https://doi.org/10.3390/nano12224048