Development of Sulfur-Doped Graphitic Carbon Nitride for Hydrogen Evolution under Visible-Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of S-Doped g-C3N4 Photocatalyst
2.3. Characterization of S-Doped g-C3N4 Photocatalyst
2.4. Photocatalytic Experiments of H2 Evolution over S-Doped g-C3N4 Photocatalyst
3. Results and Discussion
3.1. Physicochemical Properties
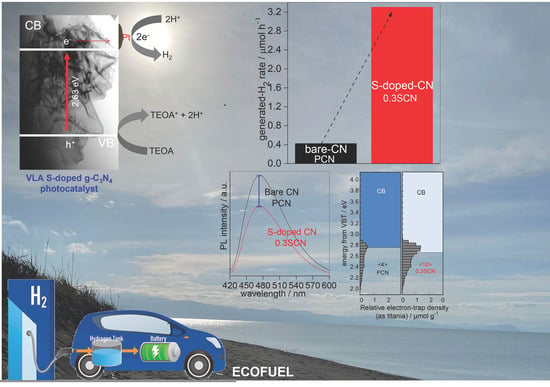
3.2. Photocatalytic H2 Generation
3.3. Improvement of Photocatalytic Performance during H2 Generation
3.4. Photocatalytic Stability and Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Kowalska, E.; Abdeldayem, H.M. The synergistic effect of anatase and brookite for photocatalytic generation of hydrogen and diclofenac degradation. J. Environ. Chem. Eng. 2021, 9, 106566. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting. Nano Res. 2020, 13, 2313–2322. [Google Scholar] [CrossRef]
- Patnaik, S.; Martha, S.; Parida, K.M. An overview of the structural, textural and morphological modulations of g-C3N4 towards photocatalytic hydrogen production. RSC Adv. 2016, 6, 46929–46951. [Google Scholar] [CrossRef]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1−XS photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338. [Google Scholar] [CrossRef]
- Wei, L.; Zeng, D.; Liu, J.; Zheng, H.; Fujita, T.; Liao, M.; Li, C.; Wei, Y. Composition-dependent activity of ZnxCd1−XSe solid solution coupled with Ni2P nanosheets for visible-light-driven photocatalytic H2 generation. J. Colloid Interface Sci. 2022, 608, 3087–3097. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Li, J. Reinforced photocatalytic H2 generation behavior of S-scheme NiO/g-C3N4 heterojunction photocatalysts with enriched nitrogen vacancies. Opt. Mater. 2023, 135, 113296. [Google Scholar] [CrossRef]
- Sun, H.; Xiao, Z.; Zhao, Z.; Zhai, S.; An, Q. Honeycomb-like porous carbon loaded with CdS/ZnS heterojunction with enhanced photocatalytic performance towards tetracycline degradation and H2 generation. Appl. Surf. Sci. 2023, 611, 155631. [Google Scholar] [CrossRef]
- Ren, X.; Tang, C.; Xu, B.; Tang, W.; Lin, B.; Yang, G. Facilitated charge transfer in ZnIn2S4@CuInP2S6 heterojunctions towards efficient photocatalytic H2 generation. Mater. Lett. 2023, 333, 133654. [Google Scholar] [CrossRef]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Ida, S.; Ishihara, T. Recent progress in two-dimensional oxide photocatalysts for water splitting. J. Phys. Chem. Lett. 2014, 5, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanomaterials for photocatalytic hydrogen production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Tang, R.; Gong, D.; Deng, Y.; Zheng, J.; Li, L.; Zhou, Z.; Yang, L.; Su, L. Environmentally-friendly carbon nanomaterials for photocatalytic hydrogen production. Chin. J. Catal. 2022, 43, 1719–1748. [Google Scholar] [CrossRef]
- Neto, A.H.; Novoselov, K. Two-dimensional crystals: Beyond graphene. Mater. Express 2011, 1, 10–17. [Google Scholar] [CrossRef]
- Bakoglidis, K.D.; Palisaitis, J.; dos Santos, R.B.; Rivelino, R.; Persson, P.O.Å.; Gueorguiev, G.K.; Hultman, L. Self-healing in carbon nitride evidenced as material inflation and superlubric behavior. ACS Appl. Mater. Interfaces 2018, 10, 16238–16243. [Google Scholar] [CrossRef] [Green Version]
- Högberg, H.; Lai, C.-C.; Broitman, E.; Ivanov, I.G.; Goyenola, C.; Näslund, L.-Å.; Schmidt, S.; Hultman, L.; Rosen, J.; Gueorguiev, G.K. Reactive sputtering of CSx thin solid films using CS2 as precursor. Vacuum 2020, 182, 109775. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Chaker, M.; Ma, D. Advancing graphitic carbon nitride-based photocatalysts toward broadband solar energy harvesting. ACS Mater. Lett. 2021, 3, 663–697. [Google Scholar] [CrossRef]
- Akhundi, A.; Zaker Moshfegh, A.; Habibi-Yangjeh, A.; Sillanpää, M. Simultaneous dual-functional photocatalysis by g-C3N4-based nanostructures. ACS EST Eng. 2022, 2, 564–585. [Google Scholar] [CrossRef]
- Ng, C.H.; Teo, S.H.; Mansir, N.; Islam, A.; Joseph, C.G.; Hayase, S.; Taufiq-Yap, Y.H. Recent advancements and opportunities of decorated graphitic carbon nitride toward solar fuel production and beyond. Sustain. Energy Fuels 2021, 5, 4457–4511. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.; Liu, S.; Wu, C. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2 evolution activity. J. Mater. Chem. A 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Liu, Z.; Wang, C.; Liu, G.; Li, Q.; Feng, X. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor. Ceram. Int. 2018, 44, 20613–20619. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, W.; Zhu, B.; Tong, F.; Pan, J.; Bai, J.; Zhou, Q.; Qin, H. Template-free one-step synthesis of g-C3N4 nanosheets with simultaneous porous network and S-doping for remarkable visible-light-driven hydrogen evolution. ACS Sustain. Chem. Eng. 2019, 7, 5801–5807. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Zhang, H.; Li, Z.; Wen, Y. One-pot synthesis of porous g-C3N4 nanosheets with enhanced photocatalytic activity under visible light. Diam. Relat. Mater. 2021, 116, 108416. [Google Scholar] [CrossRef]
- Govind, A.; Bharathi, P.; Mathankumar, G.; Mohan, M.K.; Archana, J.; Harish, S.; Navaneethan, M. Enhanced charge transfer in 2D carbon- rich g-C3N4 nanosheets for highly sensitive NO2 gas sensor applications. Diam. Relat. Mater. 2022, 128, 109205. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Xu, L.; Xu, G.; Jian, Y.; Hu, F. Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: Role of high-valent iron-oxo species and Fe–Nx sites. Chem. Eng. J. 2022, 427, 130803. [Google Scholar] [CrossRef]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4. Chemosphere 2022, 286, 131765. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, P.; Li, X.; Wu, G.; Zheng, H.; Li, C.; Fang, L.; Jiang, H. π-Conjugation extension and defects introduction into g-C3N4 by phenanthroline molecular doping to form a metal-free electrochemical sensor towards effective 4-NP detection. Diam. Relat. Mater. 2021, 119, 108557. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.R.; Karthikeyan, S.; Ohtani, B.; Sasaki, K. Fabrication and characterization of ternary sepiolite/g-C3N4/Pd composites for improvement of photocatalytic degradation of ciprofloxacin under visible light irradiation. J. Colloid Interface Sci. 2020, 577, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huang, H.; Yu, S.; Zhang, D.; Huang, H.; Zhang, Y. Role of transition metal oxides in g-C3N4-based heterojunctions for photocatalysis and supercapacitors. J. Energy Chem. 2022, 64, 214–235. [Google Scholar] [CrossRef]
- Guo, H.; Shu, Z.; Chen, D.; Tan, Y.; Zhou, J.; Meng, F.; Li, T. One-step synthesis of S-doped g-C3N4 nanosheets for improved visible-light photocatalytic hydrogen evolution. Chem. Phys. 2020, 533, 110714. [Google Scholar] [CrossRef]
- Iqbal, W.; Yang, B.; Zhao, X.; Rauf, M.; Mohamed, I.M.A.; Zhang, J.; Mao, Y. Facile one-pot synthesis of mesoporous g-C3N4 nanosheets with simultaneous iodine doping and N-vacancies for efficient visible-light-driven H2 evolution performance. Catal. Sci. Technol. 2020, 10, 549–559. [Google Scholar] [CrossRef]
- Fei, B.; Tang, Y.; Wang, X.; Dong, X.; Liang, J.; Fei, X.; Xu, L.; Song, Y.; Zhang, F. One-pot synthesis of porous g-C3N4 nanomaterials with different morphologies and their superior photocatalytic performance. Mater. Res. Bull. 2018, 102, 209–217. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Liu, Z.; Zhu, Z.; Tang, X.; Wang, Y. Study on optical properties of alkali metal doped g-C3N4 and their photocatalytic activity for reduction of CO2. Chem. Phys. Lett. 2020, 751, 137467. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Zhang, W.; Peng, Z.; Xie, H.; Wang, Y.; Guo, X.; Zhang, M.; Li, R.; Huang, Y. Template-free preparation of non-metal (B, P, S) doped g-C3N4 tubes with enhanced photocatalytic H2O2 generation. J. Mater. Sci. Technol. 2021, 95, 127–135. [Google Scholar] [CrossRef]
- Li, X.; Gan, X. Photo-fenton degradation of multiple pharmaceuticals at low concentrations via Cu-doped-graphitic carbon nitride (g-C3N4) under simulated solar irradiation at a wide PH range. J. Environ. Chem. Eng. 2022, 10, 108290. [Google Scholar] [CrossRef]
- Li, X.; Feng, D.; He, X.; Qian, D.; Nasen, B.; Qi, B.; Fan, S.; Shang, J.; Cheng, X. Z-scheme heterojunction composed of Fe doped g-C3N4 and MoS2 for efficient ciprofloxacin removal in a photo-assisted peroxymonosulfate system. Sep. Purif. Technol. 2022, 303, 122219. [Google Scholar] [CrossRef]
- Alizadeh, T.; Kadkhodayan, H. Fabrication of a highly efficient multilayer microwaves insulator based on CuFe2O4/(Pb1−xLax)/(Zr1−yTiy)O3 (composite: 7/60/40) (0 ≤ x, y ≤ 1) and Cu-doped g-C3N4 nanograins. J. Phys. Chem. Solids 2022, 170, 110941. [Google Scholar] [CrossRef]
- Jigyasa; Pratibha; Rajput, J.K. Alkali metal (Na/K) doped graphitic carbon nitride (g-C3N4) for highly selective and sensitive electrochemical sensing of nitrite in water and food samples. J. Electroanal. Chem. 2020, 878, 114605. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-light-driven photocatalytic degradation of dye and antibiotics by activated biochar composited with K+ doped g-C3N4: Effects, mechanisms, actual wastewater treatment and disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, L.; Tang, Y.; Zhang, X. A Facile Pt-doped g-C3N4 photocatalytic biosensor for visual detection of superoxide dismutase in serum samples. Sens. Actuators B Chem. 2020, 318, 128238. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Khan, A.; Al-Assiri, M.S. Au nanoparticles-doped g-C3N4 nanocomposites for enhanced photocatalytic performance under visible light illumination. Ceram. Int. 2020, 46, 22090–22101. [Google Scholar] [CrossRef]
- Zhan, X.; Zhao, Y.; Sun, Y.; Lei, C.; Wang, H.; Shi, H. Pyridazine doped g-C3N4 with nitrogen defects and spongy structure for efficient tetracycline photodegradation and photocatalytic h2 evolution. Chemosphere 2022, 307, 136087. [Google Scholar] [CrossRef]
- Duan, L.; Li, G.; Zhang, S.; Wang, H.; Zhao, Y.; Zhang, Y. Preparation of S-doped g-C3N4 with C vacancies using the desulfurized waste liquid extracting salt and its application for NOx removal. Chem. Eng. J. 2021, 411, 128551. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q. (Max); Cheng, H.-M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable synthesis of mesoporous sulfur-doped carbon nitride materials for enhanced visible light photocatalytic degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Guan, K.; Li, J.; Lei, W.; Wang, H.; Tong, Z.; Jia, Q.; Zhang, H.; Zhang, S. Synthesis of sulfur doped g-C3N4 with enhanced photocatalytic activity in molten salt. J. Mater. 2021, 7, 1131–1142. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Bahnemann, D.W. Photodegradation of 4-aminoantipyrine over nano-titania heterojunctions using solar and LED irradiation sources. J. Environ. Chem. Eng. 2019, 7, 102797. [Google Scholar] [CrossRef]
- Nitta, A.; Takase, M.; Takashima, M.; Murakami, N.; Ohtani, B. A fingerprint of metal-oxide powders: Energy-resolved distribution of electron traps. Chem. Comm. 2016, 52, 12096–12099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Mittraphab, Y.; Shimizu, K.; Ohtani, B.; Sasaki, K. Fabrication of graphitic carbon nitride/ZnTi-mixed metal oxide heterostructure: Robust photocatalytic decomposition of ciprofloxacin. J. Alloys Compd. 2022, 906, 164294. [Google Scholar] [CrossRef]
- Du, F.; Lai, Z.; Tang, H.; Wang, H.; Zhao, C. Construction of dual Z-scheme Bi2WO6/g-C3N4/black phosphorus quantum dots composites for effective bisphenol A degradation. J. Environ. Sci. 2023, 124, 617–629. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Zhao, Z.; Yan, J.; Xu, Y.; Song, Y.; Ji, H.; Xu, H.; Li, H. Hydrothermal synthesis of mpg-C3N4 and Bi2WO6 nest-like structure nanohybrids with enhanced visible light photocatalytic activities. RSC Adv. 2017, 7, 38682–38690. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Hao, X.; Li, H.; Zhang, Z.; Wang, Y.; Li, J.; Wang, Z.; Li, E. Ultrasound-assisted construction of a Z-scheme heterojunction with g-C3N4 nanosheets and flower-like Bi2WO6 microspheres and the photocatalytic activity in the coupling reaction between alcohols and amines under visible light irradiation. Mol. Catal. 2020, 494, 111122. [Google Scholar] [CrossRef]
- Deng, L.; Sun, J.; Sun, J.; Wang, X.; Shen, T.; Zhao, R.; Zhang, Y.; Wang, B. Improved performance of photosynthetic H2O2 and photodegradation by K-, P-, O-, and S-co-doped g-C3N4 with enhanced charge transfer ability under visible light. Appl. Surf. Sci. 2022, 597, 153586. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Bo, Q.; Xu, X.; Zhang, D. one-step preparation of sulfur-doped porous g-C3N4 for enhanced visible light photocatalytic performance. Dalton Trans. 2020, 49, 8041–8050. [Google Scholar] [CrossRef]
- Xie, L.; Dai, Y.; Zhou, Y.; Chang, X.; Yin, L. Sulfur (VI) modified graphite carbon nitride nanosheets with chrysanthemum-like structure and enhanced photocatalytic activity. Chem. Phys. Lett. 2018, 693, 1–7. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Yu, J.; Shen, Y.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. Dissolution and liquid crystals phase of 2D polymeric carbon nitride. J. Am. Chem. Soc. 2015, 137, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ou, H.; Fang, Y.; Wang, X. A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew. Chem. Int. Ed. 2017, 56, 3992–3996. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Zhou, C.; Xiong, W.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Gu, P.; Ma, R.; Wen, T.; Zhao, G.; Li, L.; Ai, Y.; Hu, C.; Wang, X. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies. Appl. Catal. B 2019, 248, 1–10. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Porous g-C3N4 with nitrogen defects and cyano groups for excellent photocatalytic nitrogen fixation without co-catalysts. J. Colloid Interface Sci. 2019, 556, 206–213. [Google Scholar] [CrossRef]
- Qin, H.; Lv, W.; Bai, J.; Zhou, Y.; Wen, Y.; He, Q.; Tang, J.; Wang, L.; Zhou, Q. Sulfur-doped porous graphitic carbon nitride heterojunction hybrids for enhanced photocatalytic H2 evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Iqbal, W.; Dong, C.; Xing, M.; Tan, X.; Zhang, J. Eco-friendly one-pot synthesis of well-adorned mesoporous g-C3N4 with efficiently enhanced visible light photocatalytic activity. Catal. Sci. Technol. 2017, 7, 1726–1734. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Tang, X.; Reeti, K.; Huo, P.; Wong, J.W.-C.; Zhao, J. Sulfur-doped g-C3N4 for efficient photocatalytic CO2 reduction: Insights by experiment and first-principles calculations. Catal. Sci. Technol. 2021, 11, 1725–1736. [Google Scholar] [CrossRef]
- Praus, P.; Smýkalová, A.; Foniok, K.; Velíšek, P.; Cvejn, D.; Žádný, J.; Storch, J. Post-Synthetic Derivatization of Graphitic Carbon Nitride with Methanesulfonyl Chloride: Synthesis, Characterization and Photocatalysis. Nanomaterials 2020, 10, 193. [Google Scholar] [CrossRef]
- Fang, Y.; Li, X.; Wang, Y.; Giordano, C.; Wang, X. Gradient sulfur doping along polymeric carbon nitride films as visible light photoanodes for the enhanced water oxidation. Appl. Catal. B 2020, 268, 118398. [Google Scholar] [CrossRef]
- Van, K.N.; Huu, H.T.; Nguyen Thi, V.N.; Le Thi, T.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Tran, D.L.; Vasseghian, Y. Facile construction of S-scheme SnO2/g-C3N4 photocatalyst for improved photoactivity. Chemosphere 2022, 289, 133120. [Google Scholar] [CrossRef] [PubMed]
- Raziq, F.; Humayun, M.; Ali, A.; Wang, T.; Khan, A.; Fu, Q.; Luo, W.; Zeng, H.; Zheng, Z.; Khan, B.; et al. Synthesis of S-Doped porous g-C3N4 by using ionic liquids and subsequently coupled with Au-TiO2 for exceptional cocatalyst-free visible-light catalytic activities. Appl. Catal. B 2018, 237, 1082–1090. [Google Scholar] [CrossRef]
- Lin, Y.R.; Dizon, G.V.C.; Yamada, K.; Liu, C.Y.; Venault, A.; Lin, H.Y.; Yoshida, M.; Hu, C. Sulfur-doped g-C3N4 nanosheets for photocatalysis: Z-scheme water splitting and decreased biofouling. J. Colloid Interface Sci. 2020, 567, 202–212. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Cho, M.H. Sulfur-doped-graphitic-carbon nitride (S-g-C3N4) for low cost electrochemical sensing of hydrazine. J. Alloys Compd. 2020, 816, 152522. [Google Scholar] [CrossRef]
- Kadam, A.N.; Moniruzzaman, M.; Lee, S.-W. Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes. Molecule 2019, 24, 450. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Huang, Y.; Koodail, R.T.; Liu, G.; Zeng, Y.; Meng, Q.; Yuan, M. Synthesis of Sulfur-Doped 2D Graphitic Carbon Nitride Nanosheets for Efficient Photocatalytic Degradation of Phenol and Hydrogen Evolution. ACS Appl. Mater. Interfaces 2020, 12, 12656–12667. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Pawar, R.R.; Xiong, Y.; Dabo, I.; Ohtani, B.; Kim, Y.; Song, J.T.; Ishihara, T.; Sasaki, K. Energy-resolved distribution of electron traps for O/S-doped carbon nitrides by reversed double-beam photoacoustic spectroscopy and the photocatalytic reduction of Cr(vi). Chem. Commun. 2020, 56, 3793–3796. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core–shell photocatalysts. J. Mater. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Shen, Q.; Li, N.; Bibi, R.; Richard, N.; Liu, M.; Zhou, J.; Jing, D. Incorporating nitrogen defects into novel few-layer carbon nitride nanosheets for enhanced photocatalytic H2 production. Appl. Surf. Sci. 2020, 529, 147104. [Google Scholar] [CrossRef]
- Wei, L.-F.; Zheng, X.-J.; Zhang, Z.-H.; Wei, Y.-J.; Xie, B.; Wei, M.-B.; Sun, X.-L. A systematic study of photocatalytic H2 production from propionic acid solution over Pt/TiO2 photocatalyst. Int. J. Energy Res. 2012, 36, 75–86. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Bahnemann, D.; Henglein, A.; Lilie, J.; Spanhel, L. Flash photolysis observation of the absorption spectra of trapped positive holes and electrons in colloidal titanium dioxide. J. Phys. Chem. 1984, 88, 709–711. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Kandiel, T.A.; Hussein, F.H.; Dillert, R.; Bahnemann, D.W. Solvent-free hydrothermal synthesis of anatase TiO2 nanoparticles with enhanced photocatalytic hydrogen production activity. Appl. Catal. A-Gen. 2013, 466, 32–37. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Rosman, N.N.; Yunus, R.M.; Shah, N.R.A.M.; Shah, R.M.; Arifin, K.; Minggu, L.J.; Ludin, N.A. An overview of co-catalysts on metal oxides for photocatalytic water splitting. Int. J. Energy Res. 2022, 46, 11596–11619. [Google Scholar] [CrossRef]
- Bowker, M.; O’Rourke, C.; Mills, A. The Role of Metal Nanoparticles in Promoting Photocatalysis by TiO2. Top. Curr. Chem. 2022, 380, 17. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Makise, Y.; Shuto, T.; Hu, J.; Tominaga, A.; Shironita, S.; Tanabe, S. Immobilization of noble metal nanoparticles on the surface of TiO2 by the sonochemical method: Photocatalytic production of hydrogen from an aqueous solution of ethanol. Ultrason. Sonochem. 2007, 14, 387–392. [Google Scholar] [CrossRef]
- Luna, A.L.; Dragoe, D.; Wang, K.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Bahena Uribe, D.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic hydrogen evolution using Ni–Pd/TiO2: Correlation of light absorption, charge-carrier dynamics, and quantum efficiency. J. Phys. Chem. C 2017, 121, 14302–14311. [Google Scholar] [CrossRef]
- Kisch, H. On the problem of comparing rates or apparent quantum yields in heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Bi, X.; Yu, S.; Liu, E.; Liu, L.; Zhang, K.; Zang, J.; Zhao, Y. Construction of g-C3N4/TiO2 nanotube arrays Z-scheme heterojunction to improve visible light catalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125193. [Google Scholar] [CrossRef]














| Materials | Light Source | Application | Activity | Ref. | |
|---|---|---|---|---|---|
| Raw Material | Sulfur Source | ||||
| thiourea | 300-W Xe lamp, λ > 400 nm; cut-off filter Y-42 | H2 generation | 5.3 × higher activity of doped CN | [25] | |
| dicyandiamide | H2S | 300-W Xe lamp, λ > 400 nm; cut-off filter Y-42 | H2 generation | 8 × higher activity of doped CN | [47] |
| melamine | thiourea | 300-W Xe lamp | CO2 reduction | 1.4 × higher activity of doped CN | [48] |
| thiourea | 300-W halogen lamp; λ > 400 nm | methyl orange degradation | 9.4 × higher activity of doped CN | [49] | |
| thiourea | 500-W Xe lamp | N2 fixation | 2.8 × higher activity of doped CN | [50] | |
| dicyandiamide | trithiocyanuric acid | 300-W Xe lamp | tetracycline degradation | 20 × higher activity of doped CN | [51] |
| urea | ammonium sulfate | 450-W Xe lamp, λ > 400 nm; water IR filter, cold mirror and cut-off filter (Y-42) | H2 generation | 8 × higher activity of doped CN | This work |
| Catalyst Code | AS/Urea Molar Ratio | SSA/ m2 g−1 | PS/ nm | PV/ cm3 g−1 | AE/ nm | Eg/ eV |
|---|---|---|---|---|---|---|
| PCN | 0.0 | 16.8 | 0.75–1.4 | 0.0014 | 441.1 | 2.81 |
| 0.1SCN | 0.1 | 57.4 | 3.9–6.7 | 0.19 | 459.4 | 2.78 |
| 0.2SCN | 0.2 | 65.3 | 5.1–7.5 | 0.25 | 465.1 | 2.72 |
| 0.3SCN | 0.3 | 73.8 | 5.5–8.1 | 1.3 | 476.4 | 2.63 |
| 0.4SCN | 0.4 | 68.4 | 5.3–7.9 | 0.98 | 469.3 | 2.67 |
| XPS Data | Photocatalyst | |||
|---|---|---|---|---|
| PCN | 0.3SCN | |||
| Elemental composition | atomic % | N 1s | 55.40 | 53.10 |
| C 1s | 42.60 | 43.28 | ||
| O 1s | 2.00 | 3.21 | ||
| S 2p | - | 0.41 | ||
| atomic ratio | N/C | 1.30 | 0.047 | |
| O/C | 1.23 | 0.074 | ||
| Core-levels (BE/eV) | N 1s | Pyridinic-N: N–(C)2; N–C=N | 397.3 | 397.3 |
| Pyrrolic-N: N–(C)3 | 398.4 | 398.6 | ||
| Graphitic-N: C–NHx | 400.4 | 400.4 | ||
| π-excitation | 404.2 | 404.2 | ||
| C 1s | C–C/C=C | 284.4 | 284.4 | |
| C–NHx | 286.7 | 286.7 | ||
| N–C=N | 287.8 | 287.8 | ||
| C–(N)3 | 288.4 | 288.4 | ||
| π-excitation | 293.1 | 293.1 | ||
| O 1s | O–H/adsorbed water | 532.1 | 532.1 | |
| C–N–O | 534.2 | 534.2 | ||
| S 2p | S–C | - | 164.2 | |
| Photocatalyst Code | H2 Amount/μmol | H2 Rate/μmol h−1 |
|---|---|---|
| PCN | 1.66 | 0.42 |
| 0.1SCN | 9.00 | 2.24 |
| 0.2SCN | 11.20 | 2.82 |
| 0.3SCN | 13.21 | 3.31 |
| 0.4SCN | 12.12 | 3.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, T.M.; El-Sheikh, S.M.; Endo-Kimura, M.; Wang, K.; Ohtani, B.; Kowalska, E. Development of Sulfur-Doped Graphitic Carbon Nitride for Hydrogen Evolution under Visible-Light Irradiation. Nanomaterials 2023, 13, 62. https://doi.org/10.3390/nano13010062
Khedr TM, El-Sheikh SM, Endo-Kimura M, Wang K, Ohtani B, Kowalska E. Development of Sulfur-Doped Graphitic Carbon Nitride for Hydrogen Evolution under Visible-Light Irradiation. Nanomaterials. 2023; 13(1):62. https://doi.org/10.3390/nano13010062
Chicago/Turabian StyleKhedr, Tamer M., Said M. El-Sheikh, Maya Endo-Kimura, Kunlei Wang, Bunsho Ohtani, and Ewa Kowalska. 2023. "Development of Sulfur-Doped Graphitic Carbon Nitride for Hydrogen Evolution under Visible-Light Irradiation" Nanomaterials 13, no. 1: 62. https://doi.org/10.3390/nano13010062