CeO2-Azacrown Conjugate as a Nanoplatform for Combined Radiopharmaceuticals
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
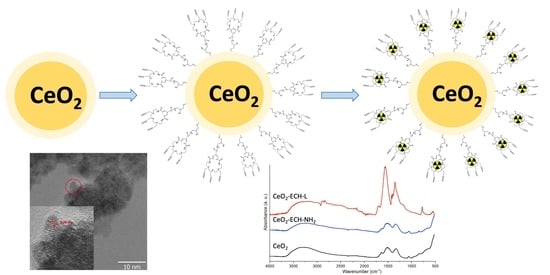
2.2. Nanoceria Synthesis and Surface Functionalization
2.3. Characterization of Modified Nanoparticles
2.4. Stability in Buffer Solutions
3. Results and Discussion
3.1. Synthesis and Functionalization of CeO2 Nanoparticles
3.2. Stability in Buffer Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goel, S.; England, C.G.; Chen, F.; Cai, W. Positron Emission Tomography and Nanotechnology: A Dynamic Duo for Cancer Theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the Intracellular Delivery of Nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Q.; Zeng, J.; Gu, Z.; Gao, M. Radiolabeling Nanomaterials for Multimodality Imaging: New Insights into Nuclear Medicine and Cancer Diagnosis. Biomaterials 2020, 228, 119553. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Zeng, M.; Parra-Robert, M.; Fernández-Varo, G.; Morales-Ruiz, M.; Jiménez, W.; Puntes, V.; Casals, G. Cerium Oxide Nanoparticles: Advances in Biodistribution, Toxicity, and Preclinical Exploration. Small 2020, 16, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.; Singh, S.; Self, W.; Tyler, R.; Seal, S.; Reilly, C.M. Bio-Distribution and in Vivo Antioxidant Effects of Cerium Oxide Nanoparticles in Mice. Environ. Toxicol. 2013, 28, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.L.; Estevez, A.Y.; DeCoteau, W.; Vangellow, S.; Ribeiro, S.; Chiarenzelli, J.; Hays-Erlichman, B.; Erlichman, J.S. Variable in Vivo and in Vitro Biological Effects of Cerium Oxide Nanoparticle Formulations. Front. Pharmacol. 2020, 10, 1599. [Google Scholar] [CrossRef]
- An, Z.; Yan, J.; Zhang, Y.; Pei, R. Applications of Nanomaterials for Scavenging Reactive Oxygen Species in the Treatment of Central Nervous System Diseases. J. Mater. Chem. B 2020, 8, 8748–8767. [Google Scholar] [CrossRef]
- Niu, J.; Azfer, A.; Rogers, L.M.; Wang, X.; Kolattukudy, P.E. Cardioprotective Effects of Cerium Oxide Nanoparticles in a Transgenic Murine Model of Cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological Potential of Cerium Oxide Nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Popov, A.L.; Zaichkina, S.I.; Popova, N.R.; Rozanova, O.M.; Romanchenko, S.P.; Ivanova, O.S.; Smirnov, A.A.; Mironova, E.V.; Selezneva, I.I.; Ivanov, V.K. Radioprotective Effects of Ultra-Small Citrate-Stabilized Cerium Oxide Nanoparticles in Vitro and in Vivo. RSC Adv. 2016, 6, 106141–106149. [Google Scholar] [CrossRef]
- Wu, Y.; Ta, H.T. Different Approaches to Synthesising Cerium Oxide Nanoparticles and Their Corresponding Physical Characteristics, and ROS Scavenging and Anti-Inflammatory Capabilities. J. Mater. Chem. B 2021, 9, 7291–7301. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Mishra, S.; Manna, K.; Das Saha, K.; Mukherjee, S.; Roy, S. Pro-Oxidant Therapeutic Activities of Cerium Oxide Nanoparticles in Colorectal Carcinoma Cells. ACS Omega 2020, 5, 9714–9723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria Exhibit Redox State-Dependent Catalase Mimetic Activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.R.B.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium Oxide Nanoparticles: Properties, Biosynthesis and Biomedical Application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef] [PubMed]
- Plakhova, T.V.; Romanchuk, A.Y.; Butorin, S.M.; Konyukhova, A.D.; Egorov, A.V.; Shiryaev, A.A.; Baranchikov, A.E.; Dorovatovskii, P.V.; Huthwelker, T.; Gerber, E.; et al. Towards the Surface Hydroxyl Species in CeO2 Nanoparticles. Nanoscale 2019, 11, 18142–18149. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size Dependency Variation in Lattice Parameter and Valency States in Nanocrystalline Cerium Oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Wang, J.; Luo, B.; Li, X.; Lu, W.; Yang, J.; Hu, Y.; Huang, P.; Wen, S. Inhibition of Cancer Growth in Vitro and in Vivo by a Novel ROS-Modulating Agent with Ability to Eliminate Stem-like Cancer Cells. Cell Death Dis. 2017, 8, e2887. [Google Scholar] [CrossRef]
- Stone, W.L.; Krishnan, K.; Campbell, S.E.; Palau, V.E. The Role of Antioxidants and Pro-Oxidants in Colon Cancer. World J. Gastrointest. Oncol. 2014, 6, 55. [Google Scholar] [CrossRef]
- Damle, M.A.; Shetty, V.G.; Jakhade, A.P.; Kaul-Ghanekar, R.; Chikate, R.C. Bi-Functional Nature of Nanoceria: Pro-Drug and Drug-Carrier Potentiality towards Receptor-Mediated Targeting of Doxorubicin. New J. Chem. 2020, 44, 17013–17026. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Spivak, N.Y.; Ivanov, V.K. Advances and Prospects of Using Nanocrystalline Ceria in Cancer Theranostics. Russ. J. Inorg. Chem. 2014, 59, 1556–1575. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Bakht, M.K.; Sadeghi, M.; Tenreiro, C. A Novel Technique for Simultaneous Diagnosis and Radioprotection by Radioactive Cerium Oxide Nanoparticles: Study of Cyclotron Production of 137mCe. J. Radioanal. Nucl. Chem. 2012, 292, 53–59. [Google Scholar] [CrossRef]
- Bakht, M.K.; Hosseini, V.; Honarpisheh, H. Radiolabeled Nanoceria Probes May Reduce Oxidative Damages and Risk of Cancer: A Hypothesis for Radioisotope-Based Imaging Procedures. Med. Hypotheses 2013, 81, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Nuclear Structure and Decay Data. Available online: https://nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (accessed on 28 June 2022).
- Chakravarty, R.; Shukla, R.; Ram, R.; Venkatesh, M.; Dash, A.; Tyagi, A.K. Nanoceria-PAN Composite-Based Advanced Sorbent Material: A Major Step Forward in the Field of Clinical-Grade 68Ge/68Ga Generator. ACS Appl. Mater. Interfaces 2010, 2, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Enrique, M.A.; Mariana, O.R.; Mirshojaei, S.F.; Ahmadi, A. Multifunctional Radiolabeled Nanoparticles: Strategies and Novel Classification of Radiopharmaceuticals for Cancer Treatment. J. Drug Target. 2015, 23, 191–201. [Google Scholar] [CrossRef]
- Patil, S.; Reshetnikov, S.; Haldar, M.K.; Seal, S.; Mallik, S. Surface-Derivatized Nanoceria with Human Carbonic Anhydrase II Inhibitors and Fluorophores: A Potential Drug Delivery Device. J. Phys. Chem. C 2007, 111, 8437–8442. [Google Scholar] [CrossRef]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Ma, D.; Abdulla, A.; Brechbiel, M.W. α-Particle Radioimmunotherapy of Disseminated Peritoneal Disease Using a 212Pb-Labeled Radioimmunoconjugate Targeting HER2. Cancer Biother. Radiopharm. 2005, 20, 557–568. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential Cytarabine and α-Particle Immunotherapy with Bismuth-213–Lintuzumab (HuM195) for Acute Myeloid Leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef] [Green Version]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of Carcinoma in Situ of the Urinary Bladder with an Alpha-Emitter Immunoconjugate Targeting the Epidermal Growth Factor Receptor: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef]
- Chilug, L.E.; Leonte, R.A.; Patrascu, M.E.B.; Ion, A.C.; Tuta, C.S.; Raicu, A.; Manda, G.; Niculae, D. In Vitro Binding Kinetics Study of Gold Nanoparticles Functionalized with 68Ga-DOTA Conjugated Peptides. J. Radioanal. Nucl. Chem. 2017, 311, 1485–1493. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Turker, M.Z.; Ma, K.; Zanzonico, P.; Gallazzi, F.; Shah, M.A.; Prater, A.R.; Wiesner, U.; Bradbury, M.S.; et al. Targeted Melanoma Radiotherapy Using Ultrasmall 177Lu-Labeled α-Melanocyte Stimulating Hormone-Functionalized Core-Shell Silica Nanoparticles. Biomaterials 2020, 241, 119858. [Google Scholar] [CrossRef] [PubMed]
- Egorova, B.V.; Matazova, E.V.; Mitrofanov, A.A.; Yu, A.G.; Trigub, A.L.; Zubenko, A.D.; Fedorova, O.A.; Fedorov, F.Y.; Kalmykov, S.N. Novel Pyridine-Containing Azacrownethers for the Chelation of Therapeutic Bismuth Radioisotopes: Complexation Study, Radiolabeling, Serum Stability and Biodistribution. Nucl. Med. Biol. 2018, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zubenko, A.D.; Shchukina, A.A.; Fedorova, O.A. Synthetic Approaches to the Bifunctional Chelators for Radionuclides Based on Pyridine-Containing Azacrown Compounds. Synthesis 2019, 51, 1087–1095. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Claussner, J.; Exner, J.; Feig, M.; Findeisen, S.; Hennig, C.; Kvashnina, K.O.; Naudet, D.; Prieur, D.; Rossberg, A.; et al. ROBL-II at ESRF: A Synchrotron Toolbox for Actinide Research. J. Synchrotron Radiat. 2021, 28, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Kvashnina, K.O.; Scheinost, A.C. A Johann-Type X-Ray Emission Spectrometer at the Rossendorf Beamline. J. Synchrotron Radiat. 2016, 23, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. Structural and Catalytic Properties of Lanthanide (La, Eu, Gd) Doped Ceria. J. Solid State Chem. 2011, 184, 3014–3020. [Google Scholar] [CrossRef]
- Fasolato, C.; Domenici, F.; Sennato, S.; Mura, F.; De Angelis, L.; Luongo, F.; Costantini, F.; Bordi, F.; Postorino, P. Dimensional Scale Effects on Surface Enhanced Raman Scattering Efficiency of Self-Assembled Silver Nanoparticle Clusters. Appl. Phys. Lett. 2014, 105, 073105. [Google Scholar] [CrossRef]
- Kvashnina, K.O.; Butorin, S.M.; Glatzel, P. Direct Study of the F-Electron Configuration in Lanthanide Systems. J. Anal. At. Spectrom. 2011, 26, 1265–1272. [Google Scholar] [CrossRef]
- Zasimov, P.; Amidani, L.; Retegan, M.; Walter, O.; Caciuffo, R.; Kvashnina, K.O. HERFD-XANES and RIXS Study on the Electronic Structure of Trivalent Lanthanides across a Series of Isostructural Compounds. Inorg. Chem. 2022, 61, 1817–1830. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabirova, S.; Aleshin, G.; Plakhova, T.; Zubenko, A.; Shchukina, A.; Fedorova, O.; Averin, A.; Belova, E.; Bazarkina, E.; Kvashnina, K.; et al. CeO2-Azacrown Conjugate as a Nanoplatform for Combined Radiopharmaceuticals. Nanomaterials 2022, 12, 4484. https://doi.org/10.3390/nano12244484
Khabirova S, Aleshin G, Plakhova T, Zubenko A, Shchukina A, Fedorova O, Averin A, Belova E, Bazarkina E, Kvashnina K, et al. CeO2-Azacrown Conjugate as a Nanoplatform for Combined Radiopharmaceuticals. Nanomaterials. 2022; 12(24):4484. https://doi.org/10.3390/nano12244484
Chicago/Turabian StyleKhabirova, Sofia, Gleb Aleshin, Tatiana Plakhova, Anastasia Zubenko, Anna Shchukina, Olga Fedorova, Aleksey Averin, Ekaterina Belova, Elena Bazarkina, Kristina Kvashnina, and et al. 2022. "CeO2-Azacrown Conjugate as a Nanoplatform for Combined Radiopharmaceuticals" Nanomaterials 12, no. 24: 4484. https://doi.org/10.3390/nano12244484