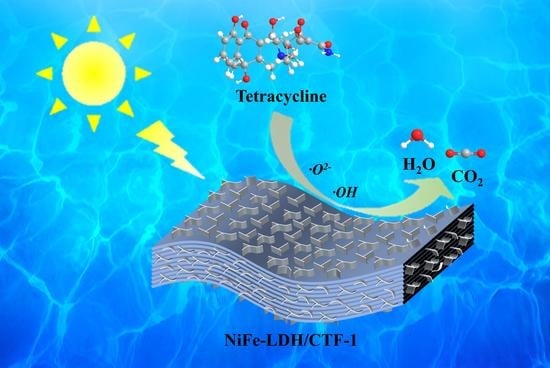
Insights into Photocatalytic Degradation Pathways and Mechanism of Tetracycline by an Efficient Z-Scheme NiFe-LDH/CTF-1 Heterojunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CTF-1
2.2. Preparation of LDH/CTF-1
2.3. Photocatalytic Activity Tests
2.4. Characterization and Performance Measurement
3. Results and Discussion
3.1. Crystal Structure, Morphology, and Physicochemical Properties
3.2. Optical and Photoelectrochemical Properties
3.3. Photocatalytic Degradation of TC
3.4. Photocatalytic Mechanism of LDH/CTF-1 Heterostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Yang, X.R.; Chen, Z.; Zhao, W.; Liu, C.X.; Qian, X.X.; Zhang, M.; Wei, G.Y.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Tan, Z.; Chen, Q.; Huang, G.; Wu, L.; Bi, J. Cobalt quantum dots as electron collectors in ultra-narrow bandgap dioxin linked covalent organic frameworks for boosting photocatalytic solar-to-fuel conversion. J. Colloid Interface Sci. 2022, 628, 573–582. [Google Scholar] [CrossRef]
- Xue, Y.; Tang, W.; Si, C.; Lu, Q.; Guo, E.; Wei, M.; Pang, Y. 0D/2D/1D silver-decorated CuPc/Bi2MoO6 Z-scheme heterojunctions enable better visible-light-driven tetracycline photocatalysis. Opt. Mater. 2022, 128, 112400. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A Hierarchical Z-Scheme CdS–WO3 Photocatalyst with Enhanced CO2 Reduction Activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, Z.; Zhao, T.; Huang, G.; Wang, J.; Bi, J. Direct Z-scheme covalent triazine-based framework/Bi2WO6 heterostructure for efficient photocatalytic degradation of tetracycline: Kinetics, mechanism and toxicity. J. Water Process Eng. 2022, 49, 103021. [Google Scholar] [CrossRef]
- Wu, S.; Xu, Z.; Zhang, J.; Zhu, M. Recent Progress on Metallic Bismuth-Based Photocatalysts: Synthesis, Construction, and Application in Water Purification. Solar RRL 2021, 5, 2100668. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Li, X.; Garlisi, C.; Guan, Q.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L. A review of material aspects in developing direct Z-scheme photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, L.; Xu, H.; Xiong, Y. 2D Polymers as Emerging Materials for Photocatalytic Overall Water Splitting. Adv. Mater. 2018, 30, 1801955. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, Z.; Yang, R.; Huang, G.; Bi, J. Integrating polyarylether-COFs with TiO2 nanofibers for enhanced visible-light-driven CO2 reduction in artificial photosynthesis. Appl. Surf. Sci. 2022, 605, 154605. [Google Scholar] [CrossRef]
- Lin, G.; Sun, L.; Huang, G.; Chen, Q.; Fang, S.; Bi, J.; Wu, L. Direct Z-scheme copper cobaltite/covalent triazine-based framework heterojunction for efficient photocatalytic CO2 reduction under visible light. Sustain. Energy Fuels 2021, 5, 732–739. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: A review. Chem. Eng. J. 2020, 392, 123684. [Google Scholar] [CrossRef]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Xiao, R.; Ye, S.; Chen, M.; Lai, C.; Xu, P.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, J.; Xu, B.; Chen, Q.; Huang, G.; Bi, J. An Artificial Photosystem of Metal-Insulator-CTF Nanoarchitectures for Highly Efficient and Selective CO2 Conversion to CO. ChemSusChem 2022, 15, e202201107. [Google Scholar] [CrossRef]
- Bi, J.; Fang, W.; Li, L.; Wang, J.; Liang, S.; He, Y.; Liu, M.; Wu, L. Covalent Triazine-Based Frameworks as Visible Light Photocatalysts for the Splitting of Water. Macromol. Rapid Commun. 2015, 36, 1799–1805. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, P.; Zhao, J. 2D covalent triazine framework: A new class of organic photocatalyst for water splitting. J. Mater. Chem. A 2015, 3, 7750–7758. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, P.; Chen, Q.; Fang, S.; Huang, G.; Bi, J.; Wu, L. Visible-light-driven photocatalyst based upon metal-free covalent triazine-based frameworks for enhanced hydrogen production. Catal. Sci. Technol. 2021, 11, 1874–1880. [Google Scholar] [CrossRef]
- Huang, G.; Niu, Q.; Zhang, J.; Huang, H.; Chen, Q.; Bi, J.; Wu, L. Platinum single-atoms anchored covalent triazine framework for efficient photoreduction of CO2 to CH4. Chem. Eng. J. 2022, 427, 131018. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, X.; Zheng, W.; Lee, L.Y.S. Interface Engineering of a 2D-C3N4/NiFe-LDH Heterostructure for Highly Efficient Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 24723–24733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hao, Y.; Zhang, G.; Lu, Z.; Han, S.; Li, Y.; Sun, X. Room-temperature synthetic NiFe layered double hydroxide with different anions intercalation as an excellent oxygen evolution catalyst. RSC Adv. 2015, 5, 55131–55135. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, K.; Ding, X.; Wang, S.; Zhang, C.; Liu, J.; Zhan, Z.; Cheng, G.; Li, B.; Chen, H.; et al. Controlling Monomer Feeding Rate to Achieve Highly Crystalline Covalent Triazine Frameworks. Adv. Mater. 2019, 31, 7865. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Hug, S.; Mesch, M.B.; Senker, J.; Lotsch, B.V. Phenyl-triazine oligomers for light-driven hydrogen evolution. Energy Environ. Sci. 2015, 8, 3345–3353. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, J.; Yan, T.; Zhu, R.; Yan, L.; Pei, Z. Adsorption and photocatalytic reduction of aqueous Cr(VI) by Fe3O4-ZnAl-layered double hydroxide/TiO2 composites. J. Colloid Interface Sci. 2020, 562, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D Hybrid Heterojunction for High-Performance Photocatalytic Reduction of CO2 into Renewable Fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Kuecken, S.; Acharjya, A.; Zhi, L.; Schwarze, M.; Schomaecker, R.; Thomas, A. Fast tuning of covalent triazine frameworks for photocatalytic hydrogen evolution. Chem. Commun. 2017, 53, 5854–5857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, G.; Bi, X.; Chen, X. Facile assembly of a hierarchical core@shell Fe3O4@CuMgAl-LDH (layered double hydroxide) magnetic nanocatalyst for the hydroxylation of phenol. J. Mater. Chem. A 2013, 1, 5934–5942. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Hsu, C.-N.; Kuo, D.H. Activating nickel iron layer double hydroxide for alkaline hydrogen evolution reaction and overall water splitting by electrodepositing nickel hydroxide. Chem. Eng. J. 2021, 419, 129608. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Kandi, D.; Behera, A.; Sahoo, S.; Parida, K. CdS QDs modified BiOI/Bi2MoO6 nanocomposite for degradation of quinolone and tetracycline types of antibiotics towards environmental remediation. Sep. Purif. Technol. 2020, 253, 117523. [Google Scholar] [CrossRef]
- Fang, P.; Wang, Z.; Wang, W. Enhanced photocatalytic performance of ZnTi-LDHs with morphology control. CrystEngComm 2019, 21, 7025–7031. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Zhou, C.; Waterhouse, G.I.N.; Wang, Z.; Weng, Y.; Zhang, T. Sub-3 nm Ultrafine Cu2O for Visible Light Driven Nitrogen Fixation. Angew. Chem. Int. Ed. 2021, 60, 2554–2560. [Google Scholar] [CrossRef]
- Ding, X.; Liu, H.; Chen, J.; Wen, M.; Li, G.; An, T.; Zhao, H. In situ growth of well-aligned Ni-MOF nanosheets on nickel foam for enhanced photocatalytic degradation of typical volatile organic compounds. Nanoscale 2020, 12, 9462–9470. [Google Scholar] [CrossRef]
- Li, F.; Sun, M.; Zhou, B.; Zhu, B.; Yan, T.; Du, B.; Shao, Y. Z-scheme bismuth-rich bismuth oxide iodide/bismuth oxide bromide hybrids with novel spatial structure: Efficient photocatalytic degradation of phenolic contaminants accelerated by in situ generated redox mediators. J. Colloid Interface Sci. 2022, 614, 233–246. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-Linked g-C3N4/rGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Ren, H.; Li, M.; Chen, L. MoS2 nanosheets anchored on porous ZnSnO3 cubes as an efficient visible-light-driven composite photocatalyst for the degradation of tetracycline and mechanism insight. J. Hazard. Mater. 2020, 390, 122158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, J.; Xu, Z.; Chen, Y.; Song, D. Degradation of tetracycline in a schorl/H2O2 system: Proposed mechanism and intermediates. Chemosphere 2018, 202, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Ma, B.; Liu, G.; Ma, X.; Zhang, C.; Ma, X.; Gao, M.; Xin, Y. Enhanced heterogeneous photo-Fenton-like degradation of tetracycline over CuFeO2/biochar catalyst through accelerating electron transfer under visible light. J. Environ. Manag. 2021, 285, 112093. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ma, W.; Wang, Y.; Zhu, Z.; Chen, Z.; Lu, G. A tribo-positive Fe@MoS(2)piezocatalyst for the durable degradation of tetracycline: Degradation mechanism and toxicity assessment. Environ. Sci. Nano 2020, 7, 1704–1718. [Google Scholar] [CrossRef]
- Barhoumi, N.; Olvera-Vargas, H.; Oturan, N.; Huguenot, D.; Gadri, A.; Ammar, S.; Brillas, E.; Oturan, M.A. Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite. Appl. Catal. B Environ. 2017, 209, 637–647. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, T.; Xu, Q.; Li, D.; Meng, S.; Chen, M. Perovskite oxide ultrathin nanosheets/g-C3N4 2D-2D heterojunction photocatalysts with significantly enhanced photocatalytic activity towards the photodegradation of tetracycline. Appl. Catal. B Environ. 2017, 201, 617–628. [Google Scholar] [CrossRef]
- Li, F.; Kang, Y.; Chen, M.; Liu, G.; Lv, W.; Yao, K.; Chen, P.; Huang, H. Photocatalytic degradation and removal mechanism of ibuprofen via monoclinic BiVO4 under simulated solar light. Chemosphere 2016, 150, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, W.; Niu, Z.; Qin, X.; Liu, X.; Shan, B.; Liu, Y. Structural, optical and photocatalytic properties of magnetic recoverable Mn0.6Zn0.4Fe2O4@Zn0.9Mn0.1O heterojunction prepared from waste Mn-Zn batteries. J. Environ. Manag. 2022, 302, 114120. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Wu, Y. A facile synthesis method for fabrication of LaFeO3/g-C3N4 nanocomposite as efficient visible-light-driven photocatalyst for photodegradation of RhB and 4-CP. New J. Chem. 2019, 43, 13783–13793. [Google Scholar] [CrossRef]











| Catalyst | Pore Volume (cm3 g−1 nm−1) | Average Pore Size (nm) | BET Surface Area (m2 g−1) |
|---|---|---|---|
| LDH | 0.95 | 13 | 281 |
| CTF-1-40% | 0.72 | 14 | 207 |
| CTF-1 | 0.13 | 24 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, X.; Chen, Q.; He, Y.; Pan, M.; Huang, G.; Bi, J. Insights into Photocatalytic Degradation Pathways and Mechanism of Tetracycline by an Efficient Z-Scheme NiFe-LDH/CTF-1 Heterojunction. Nanomaterials 2022, 12, 4111. https://doi.org/10.3390/nano12234111
Zhang J, Chen X, Chen Q, He Y, Pan M, Huang G, Bi J. Insights into Photocatalytic Degradation Pathways and Mechanism of Tetracycline by an Efficient Z-Scheme NiFe-LDH/CTF-1 Heterojunction. Nanomaterials. 2022; 12(23):4111. https://doi.org/10.3390/nano12234111
Chicago/Turabian StyleZhang, Jinpeng, Xiaoping Chen, Qiaoshan Chen, Yunhui He, Min Pan, Guocheng Huang, and Jinhong Bi. 2022. "Insights into Photocatalytic Degradation Pathways and Mechanism of Tetracycline by an Efficient Z-Scheme NiFe-LDH/CTF-1 Heterojunction" Nanomaterials 12, no. 23: 4111. https://doi.org/10.3390/nano12234111