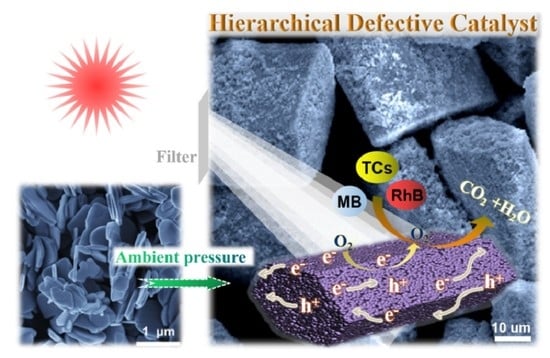
Construction of Hexagonal Prism-like Defective BiOCL Hierarchitecture for Photocatalytic Degradation of Tetracycline Hydrochloride
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Pristine BiOCl Nanosheets
2.3. Preparation of 3D-BiOCl Photocatalyst
2.4. Characterization
2.5. Photocatalytic Measurements
3. Results and Discussion
3.1. Morphology and Structure of Photocatalysts
3.2. Photocatalytic Activity and Stability of Photocatalysts under Visible Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar]
- Zhang, L.; Lu, W.; Xu, P.; Wang, H.; Wei, X.; Yao, K.; Peng, S. Plasmon-mediated activation of persulfate for efficient photodegradation of ionic liquids over Ag@ Pd core–shell nanocubes. Appl. Catal. B Environ. 2022, 301, 120751. [Google Scholar] [CrossRef]
- Zhang, J.; Jing, B.; Tang, Z.; Ao, Z.; Xia, D.; Zhu, M.; Wang, S. Experimental and DFT insights into the visible-light driving metal-free C3N5 activated persulfate system for efficient water purification. Appl. Catal. B Environ. 2021, 289, 120023. [Google Scholar] [CrossRef]
- Lee, B.-H.; Gong, E.; Kim, M.; Park, S.; Kim, H.R.; Lee, J.; Jung, E.; Lee, C.W.; Bok, J.; Jung, Y. Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O. Energy Environ. Sci. 2022, 15, 601–609. [Google Scholar] [CrossRef]
- Song, S.; Song, H.; Li, L.; Wang, S.; Chu, W.; Peng, K.; Meng, X.; Wang, Q.; Deng, B.; Liu, Q. A selective Au-ZnO/TiO2 hybrid photocatalyst for oxidative coupling of methane to ethane with dioxygen. Nat. Catal. 2021, 4, 1032–1042. [Google Scholar] [CrossRef]
- Cheng, L.; Yue, X.; Fan, J.; Xiang, Q. Site-Specific Electron-Driving Observations of CO2-to-CH4 Photoreduction on Co-doped CeO2/Crystalline Carbon Nitride S-scheme Heterojunctions. Adv. Mater. 2022, 34, 2200929. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An Inorganic/Organic S-Scheme Heterojunction H2-Production Photocatalyst and its Charge Transfer Mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef]
- Yang, N.; Zhao, Y.; Wu, P.; Liu, G.; Sun, F.; Ma, J.; Jiang, Z.; Sun, Y.; Zeng, G. Defective C3N4 frameworks coordinated diatomic copper catalyst: Towards mild oxidation of methane to C1 oxygenates. Appl. Catal. B Environ. 2021, 299, 120682. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Li, H.; Wang, X.; Wei, J.; Jiao, X.; Chen, D. Oxygen vacancy dependent photocatalytic CO2 reduction activity in liquid-exfoliated atomically thin BiOCl nanosheets. Appl. Catal. B Environ. 2021, 297, 120426. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Zhangai, C.; Cheng, T.; Wang, Y.; Zhang, B.; Dong, P.; Chen, X.; Xi, X.; Zou, Z. Layered BiOCl/H+ TiNbO 5− heterojunctions for boosting visible-light-driven photocatalytic RhB degradation. Sustain. Energy Fuels 2021, 5, 4680–4689. [Google Scholar] [CrossRef]
- Qi, S.; Liu, X.; Ma, N.; Xu, H. Construction and photocatalytic properties of WS2/MoS2/BiOCl heterojunction. Chem. Phys. Lett. 2021, 763, 138203. [Google Scholar] [CrossRef]
- Liu, G.; Wang, B.; Zhu, X.; Ding, P.; Zhao, J.; Li, H.; Chen, Z.; Zhu, W.; Xia, J. Edge-Site-Rich Ordered Macroporous BiOCl Triggers C=O Activation for Efficient CO2 Photoreduction. Small 2022, 18, 2105228. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen vacancy-mediated photocatalysis of BiOCl: Reactivity, selectivity, and perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Batmunkh, M.; Mao, H.; Li, H.; Jia, B.; Wu, S.; Liu, D.; Song, X.; Sun, Y.; Ma, T. Low-overpotential electrochemical ammonia synthesis using BiOCl-modified 2D titanium carbide MXene. Chin. Chem. Lett. 2022, 33, 394–398. [Google Scholar] [CrossRef]
- Wei, S.; Zhong, H.; Wang, H.; Song, Y.; Jia, C.; Anpo, M.; Wu, L. Oxygen vacancy enhanced visible light photocatalytic selective oxidation of benzylamine over ultrathin Pd/BiOCl nanosheets. Appl. Catal. B Environ. 2022, 305, 121032. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Ala’a, H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment, Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, G.; Li, H.; Wang, X.; Liu, X.; Shi, L.; Wei, K.; Ling, C.; Li, Z.; Wang, H. Simultaneous manipulation of bulk excitons and surface defects for ultrastable and highly selective CO2 photoreduction. Adv. Mater. 2021, 33, 2100143. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Li, R.; Wang, Y.; Wang, Y.; Fan, C. PtO/Pt4+-BiOCl with enhanced photocatalytic activity: Insight into the defect-filled mechanism. Chem. Eng. J. 2020, 395, 123954. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, Y.; Mu, H.Y.; Liu, R.H.; Hao, Y.J.; Wang, X.J.; Hildebrandt, D.; Liu, X.; Li, F.T. The simultaneous adsorption, activation and in situ reduction of carbon dioxide over Au-loading BiOCl with rich oxygen vacancies. Nanoscale 2021, 13, 2585–2592. [Google Scholar] [CrossRef]
- Tian, C.; Luo, S.; She, J.; Qing, Y.; Yan, N.; Wu, Y.; Liu, Z. Cellulose nanofibrils enable flower-like BiOCl for high-performance photocatalysis under visible-light irradiation. Appl. Surf. Sci. 2019, 464, 606–615. [Google Scholar] [CrossRef]
- Lin, E.; Huang, R.; Wu, J.; Kang, Z.; Ke, K.; Qin, N.; Bao, D. Recyclable CoFe2O4 modified BiOCl hierarchical microspheres utilizing photo, photothermal and mechanical energy for organic pollutant degradation. Nano Energy 2021, 89, 106403. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Xu, P.; Xue, W.; Lei, L.; Chen, Y.; Zhou, C.; Deng, R.; Wang, W. Topological transformation of bismuth vanadate into bismuth oxychloride: Band-gap engineering of ultrathin nanosheets with oxygen vacancies for efficient molecular oxygen activation. Chem. Eng. J. 2021, 420, 127573. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Duan, R.; Wan, Q.; Tan, X.; Su, Z.; Han, B.; Zheng, L.; Mo, G. BiOCl nanosheets with periodic nanochannels for high-efficiency photooxidation. Nano Energy 2020, 78, 105340. [Google Scholar] [CrossRef]
- Cui, D.; Wang, L.; Xu, K.; Ren, L.; Wang, L.; Yu, Y.; Du, Y.; Hao, W. Band-gap engineering of BiOCl with oxygen vacancies for efficient photooxidation properties under visible-light irradiation. J. Mater. Chem. A 2018, 6, 2193–2199. [Google Scholar] [CrossRef]
- Qin, H.; Sun, J.; Xia, D.; Xu, H.; Yu, Q.; Zheng, Y.; Shi, Y. Boosting nonradical process in BiOI/BiOCl heterostructure by interface oxygen vacancies. Chem. Eng. J. 2022, 435, 134847. [Google Scholar] [CrossRef]
- Cui, D.; Xu, K.; Dong, X.; Lv, D.; Dong, F.; Hao, W.; Du, Y.; Chen, J. Controlled hydrogenation into defective interlayer bismuth oxychloride via vacancy engineering. Commun. Chem. 2020, 3, 73. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Guo, R.-C.; Chen, Y.-F.; Xu, X.; Yang, Z.-X.; Cheng, D.-B.; Chen, H.; Qiao, Z.-Y.; Wang, H. A BiOCl nanodevice for pancreatic tumor imaging and mitochondria-targeted therapy. Nano Today 2021, 40, 101285. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Shang, H.; Wang, X.; Yang, Z.; Ai, Z.; Zhang, L. Surface hydrogen bond network spatially confined BiOCl oxygen vacancy for photocatalysis. Sci. Bull. 2020, 65, 1916–1923. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Li, G.; Qin, F.; Chen, R. Well-crystallized square-like 2D BiOCl nanoplates: Mannitol-assisted hydrothermal synthesis and improved visible-light-driven photocatalytic performance. RSC Adv. 2011, 1, 1542–1553. [Google Scholar]
- Mi, Y.; Wen, L.; Wang, Z.; Cao, D.; Fang, Y.; Lei, Y. Building of anti-restack 3D BiOCl hierarchitecture by ultrathin nanosheets towards enhanced photocatalytic activity. Appl. Catal. B Environ. 2015, 176–177, 331–337. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, L.; Zhang, M.; Li, Y.; Liu, S.; Lin, J.; Song, F.; Zhang, W.; Zhu, S.; Pan, J. Construction of a bioinspired hierarchical BiVO4/BiOCl heterojunction and its enhanced photocatalytic activity for phenol degradation. ACS Appl. Mater. Interfaces 2021, 13, 32906–32915. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, S.; Yang, S.-Z.; Li, Q.; Xiong, J.; Yang, Z.; Gu, L.; Zhang, X.; Sun, L. Enhanced photocatalytic activity induced by sp3 to sp2 transition of carbon dopants in BiOCl crystals. Appl. Catal. B Environ. 2018, 221, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Rao, F.; Zhang, W.; Hassan, Q.-U.; Liu, Z.; Gao, J.; Lu, J.; Hojamberdiev, M.; Zhu, G. Au nanoparticles loaded on hollow BiOCl microstructures boosting CO2 photoreduction. Chin. Chem. Lett. 2021, 33, 4385–4388. [Google Scholar] [CrossRef]
- Kuila, A.; Saravanan, P.; Bahnemann, D.; Wang, C. Novel Ag decorated, BiOCl surface doped AgVO3 nanobelt ternary composite with Z-scheme homojunction-heterojunction interface for high prolific photo switching, quantum efficiency and hole mediated photocatalysis. Appl. Catal. B Environ. 2021, 293, 120224. [Google Scholar] [CrossRef]
- Cao, J.; Cen, W.; Jing, Y.; Du, Z.; Chu, W.; Li, J. P-doped BiOCl for visible light photodegradation of tetracycline: An insight from experiment and calculation. Chem. Eng. J. 2022, 435, 134683. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Kalathil, S.; Nisar, A.; Lee, J.; Cho, M.H. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 2013, 5, 9238–9246. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, Q.; Yang, L.; Luo, X.; Wang, A.; Luo, S.; Dionysiou, D.D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Wang, L.; Lv, D.; Dong, F.; Wu, X.; Cheng, N.; Scott, J.; Xu, X.; Hao, W.; Du, Y. Boosting visible-light-driven photo-oxidation of BiOCl by promoted charge separation via vacancy engineering. ACS Sustain. Chem. Eng. 2019, 7, 3010–3017. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Z.; Zheng, W.; Lei, Z.; Qiu, J.; Kuang, W.; Huang, W.; Feng, C. Facile ammonium oxidation to nitrogen gas in acid wastewater by in situ photogenerated chlorine radicals. Water Res. 2021, 205, 117678. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Jiang, D.; Gao, E.; Sun, S. Photoreduction of CO2 on BiOCl nanoplates with the assistance of photoinduced oxygen vacancies. Nano Res. 2015, 8, 821–831. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Wang, W.; Li, X.; Su, Y.; Jiang, D.; Lei, X.; Sun, S. Solar-light-driven pure water splitting with ultrathin BiOCl nanosheets. Chem. Eur. J. 2015, 21, 18089–18094. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, D.; Liu, X.; Luo, Y.; Xue, H.; Huang, B.; Chen, Q.; Qian, Q. Electrospun BiOCl/Bi2Ti2O7 nanorod heterostructures with enhanced solar light efficiency in the photocatalytic degradation of tetracycline hydrochloride. ChemCatChem 2018, 10, 2496–2504. [Google Scholar] [CrossRef]
- Wang, Y.; Qiang, Z.; Zhu, W.; Yao, W.; Tang, S.; Yang, Z.; Wang, J.; Duan, J.; Ma, C.; Tan, R. BiPO4 nanorod/graphene composite heterojunctions for photocatalytic degradation of tetracycline hydrochloride. ACS Appl. Nano Mater. 2021, 4, 8680–8689. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, L.; Wang, P.; Shi, Z.; Zhang, L. Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr (VI) coexistence environment. Appl. Catal. B Environ. 2020, 262, 118308. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, Z.; Xu, Y.; Tian, T.; Zhang, J.; Pang, J.; Peng, X.; Zhang, Q.; Shao, M.; Tan, W. Visible-light-driven AgBr–TiO2-Palygorskite photocatalyst with excellent photocatalytic activity for tetracycline hydrochloride. J. Clean. Prod. 2020, 277, 124021. [Google Scholar] [CrossRef]
- Wang, H.; Liao, B.; Lu, T.; Ai, Y.; Liu, G. Enhanced visible-light photocatalytic degradation of tetracycline by a novel hollow BiOCl@ CeO2 heterostructured microspheres: Structural characterization and reaction mechanism. J. Hazard. Mater. 2020, 385, 121552. [Google Scholar] [CrossRef]
- Huang, H.; Ma, C.; Zhu, Z.; Yao, X.; Liu, Y.; Liu, Z.; Li, C.; Yan, Y. Insights into enhanced visible light photocatalytic activity of t-Se nanorods/BiOCl ultrathin nanosheets 1D/2D heterojunctions. Chem. Eng. J. 2018, 338, 218–229. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, X.; Ma, C.; Huang, H.; Yu, K.; Liu, Y.; Lu, Z.; Li, C.; Zhu, Z.; Huo, P. A 2D mesoporous photocatalyst constructed by the modification of biochar on BiOCl ultrathin nanosheets for enhancing the TC-HCl degradation activity. New J. Chem. 2020, 44, 79–86. [Google Scholar] [CrossRef]
- Guan, C.; Hou, T.; Nie, W.; Zhang, Q.; Duan, L.; Zhao, X. Facet synergy dominant Z-scheme transition in BiOCl with enhanced 1O2 generation. Chemosphere 2022, 307, 135663. [Google Scholar] [CrossRef]
- Gao, P.; Li, Z.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Construction of novel MWCNTs/Bi4O5I2 nanosheets with enhanced adsorption and photocatalytic performance for the degradation of tetracycline: Efficiency, mechanism and regeneration. Chem. Eng. J. 2022, 429, 132398. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Luo, P.; He, B.; Cao, X.; Lu, T. Iron sites on defective BiOBr nanosheets: Tailoring the molecular oxygen activation for enhanced photocatalytic organic synthesis. Nano Res. 2022, 15, 1509–1516. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Bian, K.; Zeng, W.; Jin, X.; Ouyang, R.; Xu, Y.; Dai, C.; Zhou, S.; Zhang, B. Oxygen-Deficient BiOCl Combined with L-Buthionine-Sulfoximine Synergistically Suppresses Tumor Growth through Enhanced Singlet Oxygen Generation under Ultrasound Irradiation. Small 2022, 18, 2104550. [Google Scholar] [CrossRef] [PubMed]
- D’amato, C.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Enhancement of visible-light photoactivity by polypropylene coated plasmonic Au/TiO2 for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, J.; Zhao, X.; Lin, X.; Hao, Y.; Huo, P.; Meng, M.; Yan, Y. Nitrogen defect engineering and π-conjugation structure decorated g-C3N4 with highly enhanced visible-light photocatalytic hydrogen evolution and mechanism insight. Chem. Eng. J. 2021, 425, 131844. [Google Scholar] [CrossRef]
- Li, G.; Deng, X.; Chen, P.; Wang, X.; Ma, J.; Liu, F.; Yin, S.-F. Sulphur vacancies-VS2@ C3N4 drived by in situ supramolecular self-assembly for synergistic photocatalytic degradation of real wastewater and H2 production: Vacancies taming interfacial compact heterojunction and carriers transfer. Chem. Eng. J. 2022, 433, 134505. [Google Scholar] [CrossRef]
- Jang, D.; Choi, S.; Kwon, N.H.; Jang, K.Y.; Lee, S.; Lee, T.-W.; Hwang, S.-J.; Kim, H.; Kim, J.; Park, S. Water-assisted formation of amine-bridged carbon nitride: A structural insight into the photocatalytic performance for H2 evolution under visible light. Appl. Catal. B Environ. 2022, 310, 121313. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Salehi, G. Preparation of amine functionalized g-C3N4@ H/SMOF NCs with visible light photocatalytic characteristic for 4-nitrophenol degradation from aqueous solution. J. Hazard. Mater. 2019, 365, 921–931. [Google Scholar] [CrossRef]
- Le, S.; Zhu, C.; Cao, Y.; Wang, P.; Liu, Q.; Zhou, H.; Chen, C.; Wang, S.; Duan, X. V2O5 nanodot-decorated laminar C3N4 for sustainable photodegradation of amoxicillin under solar light. Appl. Catal. B Environ. 2022, 303, 120903. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Jin, Z.; Huang, M.; Zhou, F.; Song, J.; Qu, J.; Zeng, Y.J.; Qian, P.C.; Wong, W.Y. Solar-Driven Hydrogen Generation Catalyzed by g-C3N4 with Poly (platinaynes) as Efficient Electron Donor at Low Platinum Content. Adv. Sci. 2021, 8, 2002465. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Yang, K.; Xiong, Z.; Yang, B.; Wang, M.; Tang, X.; Zang, Z.; Liu, X.; Li, B.; et al. PEDOT:PSS monolayers to enhance the hole extraction and stability of perovskite solar cells. J. Mater. Chem. A 2018, 6, 16583–16589. [Google Scholar] [CrossRef]
- Reddy, C.V.; Koutavarapu, R.; Shim, J.; Cheolho, B.; Reddy, K.R. Novel g-C3N4/Cu-doped ZrO2 hybrid heterostructures for efficient photocatalytic Cr (VI) photoreduction and electrochemical energy storage applications. Chemosphere 2022, 295, 133851. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, S.; Ma, H.; Gao, J.; Zhu, G.; Zhang, F.; Park, J.Y.; Cha, S.; Bae, J.-S.; Liu, C. Oxygen vacancy rich Bi2O4-Bi4O7-BiO2-x composites for UV–vis-NIR activated high efficient photocatalytic degradation of bisphenol A. J. Hazard. Mater. 2020, 382, 121121. [Google Scholar] [CrossRef] [PubMed]





| Types of Photocatalyst | Light Source | Concentration of TC-HCl (mg/L) | Photocatalyst Dosage (g/L) | kinetic Constants (min–1) | References |
|---|---|---|---|---|---|
| BiOCl/Bi2Ti2O7 | Simulated solar light | 50 | 1 | 0.016 | [42] |
| BiPO4 Nanorod/Graphene | UV light | 20 | 0.5 | 0.034 | [43] |
| N–TiO2/CNONV | Visble light (λ > 420 nm) | 30 | 0.4 | 0.017 | [44] |
| AgBr–TiO2–Pa | Visble light (λ > 420 nm) | 20 | 0.5 | 0.019 | [45] |
| BiOCl@CeO2 | Visble light (λ > 420 nm) | 10 | 0.5 | 0.015 | [46] |
| Se/BiOCl | Visble light (λ > 420 nm) | 10 | 0.5 | 0.022 | [47] |
| C/BiOCl | Visble light (λ > 420 nm) | 10 | 0.5 | 0.014 | [48] |
| BiOCl | Visble light (λ > 420 nm) | 10 | 0.4 | 0.007 | [49] |
| MWCNTs/Bi4O5I2 | Visble light | 20 | 0.2 | 0.012 | [50] |
| 3D–BiOCl | Visble light (λ > 420 nm) | 30 | 1 | 0.025 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Ding, Z.; Yan, F.; Li, K.; Feng, L.; Wang, H. Construction of Hexagonal Prism-like Defective BiOCL Hierarchitecture for Photocatalytic Degradation of Tetracycline Hydrochloride. Nanomaterials 2022, 12, 2700. https://doi.org/10.3390/nano12152700
Hu L, Ding Z, Yan F, Li K, Feng L, Wang H. Construction of Hexagonal Prism-like Defective BiOCL Hierarchitecture for Photocatalytic Degradation of Tetracycline Hydrochloride. Nanomaterials. 2022; 12(15):2700. https://doi.org/10.3390/nano12152700
Chicago/Turabian StyleHu, Lijun, Zhichao Ding, Fei Yan, Kuan Li, Li Feng, and Hongqing Wang. 2022. "Construction of Hexagonal Prism-like Defective BiOCL Hierarchitecture for Photocatalytic Degradation of Tetracycline Hydrochloride" Nanomaterials 12, no. 15: 2700. https://doi.org/10.3390/nano12152700