Adsorptive Removal of Naproxen from Water Using Polyhedral Oligomeric Silesquioxane (POSS) Covalent Organic Frameworks (COFs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Octa(nitrophenyl)silesquioxane (ONPS)
2.2. Synthesis of Octa(amino phenyl)silesquioxane (OAPS)
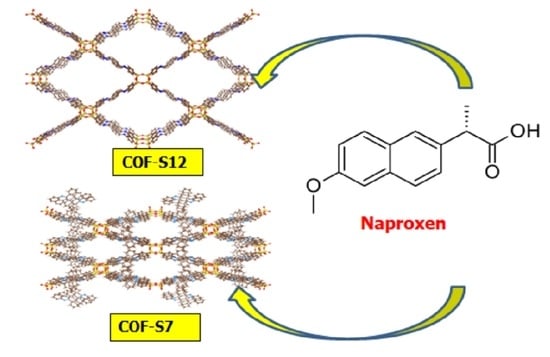
2.3. Synthesis of COF-S7
2.4. Synthesis of COF-S12
2.5. Characterization of the COF Materials
2.6. Adsorption Experiments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Chemistry: Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Waller, P.J.; Gándara, F. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Chen, L.; Furukawa, K.; Gao, J.; Nagai, A.; Nakamura, T.; Dong, Y.; Jiang, D. Photoelectric covalent organic frameworks: Converting open lattices into ordered donor-acceptor heterojunctions. J. Am. Chem. Soc. 2014, 136, 9806–9809. [Google Scholar] [CrossRef]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. Engl. 2008, 47, 8826–8830. [Google Scholar] [CrossRef]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Huang, W.; Gan, L.; Li, H.; Ma, Y.; Zhai, T. 2D layered group IIIA metal chalcogenides: Synthesis, properties and applications in electronics and optoelectronics. CrystEngComm 2016, 18, 3968–3984. [Google Scholar] [CrossRef]
- Liao, H.; Wang, H.; Ding, H.; Meng, X.; Xu, H.; Wang, B.; Ai, X.; Wang, C. A 2D porous porphyrin-based covalent organic framework for sulfur storage in lithium-sulfur batteries. J. Mater. Chem. A 2016, 4, 7416–7421. [Google Scholar] [CrossRef]
- Furukawa, H.; Liu, Z.; Zhu, H.; Zhu, C.; Suenaga, K.; Liu, Y.; Ma, Y.; Zhao, Y.; Sun, X.; Gándara, F.; et al. Weaving of organic threads into a crystalline covalent organic framework. Science 2016, 351, 365–369. [Google Scholar]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.Y.; Xu, S.Q.; Wen, Q.; Pang, Z.F.; Zhao, X. One-step construction of two different kinds of pores in a 2D covalent organic framework. J. Am. Chem. Soc. 2014, 136, 15885–15888. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Addicoat, M.; Jin, S.; Sakurai, T.; Gao, J.; Xu, H.; Irle, S.; Seki, S.; Jiang, D. Rational design of crystalline supermicroporous covalent organic frameworks with triangular topologies. Nat. Commun. 2015, 6, 7786. [Google Scholar] [CrossRef]
- Ding, S.Y.; Dong, M.; Wang, Y.W.; Chen, Y.T.; Wang, H.Z.; Su, C.Y.; Wang, W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-S.; Ding, S.Y.; An, W.K.; Wu, H.; Wang, W. Constructing Crystalline Covalent Organic Frameworks from Chiral Building Blocks. J. Am. Chem. Soc. 2016, 138, 11489–11492. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Lee, G.; Jang, M.; Jang, A.; Her, N.; Son, A.; Yoon, Y. Ultrasonic treatment of endocrine disrupting compounds, pharmaceuticals, and personal care products in water: A review. Chem. Eng. J. 2017, 327, 629–647. [Google Scholar] [CrossRef]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Son, A.; Her, N.; Zoh, K.D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Vymazal, J.; Březinová, T.; Koželuh, M.; Kule, L.; Huang, J.; Chen, Z. Occurrence, removal and environmental risk assessment of pharmaceuticals and personal care products in rural wastewater treatment wetlands. Sci. Total Environ. 2016, 566–567, 1660–1669. [Google Scholar] [CrossRef]
- Wu, X.; Conkle, J.L.; Ernst, F.; Gan, J. Treated wastewater irrigation: Uptake of pharmaceutical and personal care products by common vegetables under field conditions. Environ. Sci. Technol. 2014, 48, 11286–11293. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Du, B.; Chambliss, C.K.; Koschorreck, J.; Rüdel, H.; Quack, M.; Brooks, B.W.; Usenko, S. Occurrence of pharmaceuticals and personal care products in German fish tissue: A national study. Environ. Sci. Technol. 2012, 46, 9047–9054. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2014, 86, 2813–2848. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Kansal, S.K. Heterogeneous photocatalytic studies of analgesic and non-steroidal anti-inflammatory drugs. Appl. Catal. A Gen. 2016, 510, 134–155. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, T.; Rodil, R.; Quintana, J.B.; Triñanes, S.; Cela, R. Oxidation of non-steroidal anti-inflammatory drugs with aqueous permanganate. Water Res. 2013, 47, 3220–3230. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Noguera-oviedo, K.; Aga, D.S. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Acid, C.; Mu, S.R. Occurrence and Fate of and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar]
- Ganiyu, S.O.; Van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Nakada, N.; Shinohara, H.; Murata, A.; Kiri, K.; Managaki, S.; Sato, N.; Takada, H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007, 41, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Vona, A.; di Martino, F.; Garcia-Ivars, J.; Picó, Y.; Mendoza-Roca, J.A.; Iborra-Clar, M.I. Comparison of different removal techniques for selected pharmaceuticals. J. Water Process Eng. 2015, 5, 48–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Price, G.W.; Jamieson, R.; Burton, D.; Khosravi, K. Chemosphere Sorption and desorption of selected non-steroidal anti-inflammatory drugs in an agricultural loam-textured soil. Chemosphere 2017, 174, 628–637. [Google Scholar] [CrossRef]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound—Naproxen, carbamazepine and nonylphenol—on activated carbon. Water Res. 2008, 42, 2873–2882. [Google Scholar] [CrossRef]
- Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Silvestre-Albero, J. Assessment of naproxen adsorption on bone char in aqueous solutions using batch and fi xed-bed processes. J. Mol. Liq. 2015, 209, 187–195. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Coupling granular activated carbon adsorption with membrane bioreactor treatment for trace organic contaminant removal: Breakthrough behaviour of persistent and hydrophilic compounds. J. Environ. Manag. 2013, 119, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Jiménez, S.M.; Méndez-González, S.; Hernández-Maldonado, A. Microporous and Mesoporous Materials of transition metal incorporation and pH conditions on the adsorption of Naproxen from water. Microporous Mesoporous Mater. 2010, 132, 470–479. [Google Scholar] [CrossRef]
- Xuan, T.; Hung, V.; Thanh, S.; Choi, H. Adsorption of pharmaceuticals onto trimethylsilylated mesoporous. J. Hazard. Mater. 2013, 254–255, 345–353. [Google Scholar] [CrossRef]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209–210, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D.; Huang, N.; Zhai, L.; Xu, H.; Jiang, D. Stable Covalent Organic Frameworks for Exceptional Mercury Removal from Aqueous Solutions Stable Covalent Organic Frameworks for Exceptional Mercury Removal from Aqueous Solutions. J. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Mellah, A.; Fernandes, S.P.S.; Rodríguez, R.; Otero, J.; Paz, J.; Cruces, J.; Medina, D.D.; Djamila, H.; Espiña, B.; Salonen, L.M. Adsorption of Pharmaceutical Pollutants from Water Using Covalent Organic Frameworks. Chem. A Eur. J. 2018, 24, 10601–10605. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Qiang, H.; Hong, X.; Luo, F. Journal of Solid State Chemistry U (VI) adsorption onto covalent organic frameworks-TpPa-1. J. Solid State Chem. 2019, 277, 484–492. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Wang, J. Covalent organic frameworks as e ffi cient adsorbent for sulfamerazine removal from aqueous solution. J. Hazard. Mater. 2020, 383, 121126. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C.; Allaudeen, N.; Rajan, S.; Sophia, A.C.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater- a review. Desalin. Water Treat. 2016, 3994, 1–14. [Google Scholar] [CrossRef]
- Vitaku, E.; Dichtel, W.R. Synthesis of 2D Imine-Linked Covalent Organic Frameworks through Formal Transimination Reactions. J. Am. Chem. Soc. 2017, 139, 12911–12914. [Google Scholar] [CrossRef]
- Matsumoto, M.; Dasari, R.R.; Ji, W.; Feriante, C.H.; Parker, T.C.; Marder, S.R.; Dichtel, W.R. Rapid, Low Temperature Formation of Imine-Linked Covalent Organic Frameworks Catalyzed by Metal Triflates. J. Am. Chem. Soc. 2017, 139, 4999–5002. [Google Scholar] [CrossRef]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.; et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Jothibasu, S.; Premkumar, S.; Alagar, M.; Hamerton, I. Synthesis and characterization of a POSS-maleimide precursor for hybrid nanocomposites. High Perform. Polym. 2008, 20, 67–85. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Peng, Q.; Song, J. Preparation of polyhedral oligosilsesquioxane-based organic-inorganic hybrid porous nanocomposite by freeze-drying conditions. Asian J. Chem. 2013, 25, 1229–1232. [Google Scholar] [CrossRef]
- Tamaki, R.; Tanaka, Y.; Asuncion, M.Z.; Choi, J.; Laine, R.M. Octa (aminophenyl) silsesquioxane as a nanoconstruction site. J. Am. Chem. Soc. 2001, 123, 12416–12417. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Tamaki, R.; Kim, S.G.; Laine, R.M. Organic/Inorganic Imide Nanocomposites from Aminophenylsilsesquioxanes. Chem. Mater. 2003, 15, 3365–3375. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S. A novel photocrosslinkable polyhedral oligomeric silsesquioxane and its nanocomposites with poly(vinyl cinnamate). Chem. Mater. 2004, 16, 5141–5148. [Google Scholar] [CrossRef]
- Hoffmann, H.C.; Debowski, M.; Müller, P.; Paasch, S.; Senkovska, I.; Kaskel, S.; Brunner, E. Solid-state NMR spectroscopy of metal-organic framework compounds (MOFs). Materials 2012, 5, 2537–2572. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Balaji Shinde, D.; Kandambeth, S.; Biswal, B.P.; Banerjee, R. Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 2014, 50, 12615–12618. [Google Scholar] [CrossRef]
- Hunt, J.R.; Doonan, C.J.; LeVangie, J.D.; Côté, A.P.; Yaghi, O.M. Reticular synthesis of covalent organic borosilicate frameworks. J. Am. Chem. Soc. 2008, 130, 11872–11873. [Google Scholar] [CrossRef]
- Yahiaoui, O.; Fitch, A.N.; Hoffmann, F.; Fröba, M.; Thomas, A.; Roeser, J. 3D anionic silicate covalent organic framework with srs topology. J. Am. Chem. Soc. 2018, 140, 5330–5333. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Z.L.; Wei, L.; Zhou, B.; Tan, J.; Zhou, H.L.; Zhang, Y.B. Guest-Dependent Dynamics in a 3D Covalent Organic Framework. J. Am. Chem. Soc. 2019, 141, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Gropp, C.; Yaghi, O.M. Reticulating 1D Ribbons into 2D Covalent Organic Frameworks by Imine and Imide Linkages. J. Am. Chem. Soc. 2020, 142, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best practices for the synthesis, activation, and characterization of metal−organic frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Lippke, J.; Brosent, B.; Zons, T.V.; Virmani, E.; Lilienthal, S.; Preuße, T.; Hu, M.; Schneider, A.M.; Wuttke, S.; Behrens, P.; et al. Expanding the Group of Porous Interpenetrated Zr-Organic Frameworks (PIZOFs) with Linkers of Different Lengths. Inorg. Chem. 2017, 56, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, X.; Jiang, W.; Liu, H. Determination of nereistoxin-related insecticide via quantum-dots-doped covalent organic frameworks in a molecularly imprinted network. Microchim. Acta 2020, 187, 464. [Google Scholar] [CrossRef]
- Renzo, F.D.; Chang, S.; Clair, B.; Ruelle, J.; Beauche, J. Mesoporosity as a new parameter for understanding tension stress generation in trees. J. Exp. Bot. 2009, 60, 3023–3030. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Zou, R.; Luo, Z.; Zhang, H.; Yao, X.; Zou, R.; Zhao, Y. Covalent Organic Frameworks Formed with Two Types of Covalent Bonds Based on Orthogonal Reactions. J. Am. Chem. Soc. 2015, 1021–1024. [Google Scholar] [CrossRef]
- Ayoub, G.; Islamoglu, T.; Goswami, S.; Friščić, T.; Farha, O.K. Torsion Angle Effect on the Activation of UiO Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 15788–15794. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 2018, 335, 74–81. [Google Scholar] [CrossRef]
- Ahmed, I.; Khan, N.A.; Jhung, S.H. Graphite Oxide/Metal—Organic Framework (MIL-101): Remarkable Performance in the Adsorptive Denitrogenation of Model Fuels. Inorg. Chem. 2013, 52, 14155–14161. [Google Scholar] [CrossRef]
- Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorptive removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from water with a metal-organic framework. Chem. Eng. J. 2013, 234, 99–105. [Google Scholar] [CrossRef]
- Khan, N.A.; Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal-organic frameworks. J. Hazard. Mater. 2015, 282, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Choi, E.J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. A remarkable adsorbent for removal of contaminants of emerging concern from water: Porous carbon derived from metal azolate framework-6. J. Hazard. Mater. 2017, 340, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, D.; Huang, H.; Yang, Q.; Zhong, C. Efficient capture of nitrobenzene from waste water using metal-organic frameworks. Chem. Eng. J. 2014, 246, 142–149. [Google Scholar] [CrossRef]
- Feng, B.; Shen, W.; Shi, L.; Qu, S. Adsorption of hexavalent chromium by polyacrylonitrile-based porous carbon from aqueous solution. R. Soc. Open Sci. 2018, 5, 171662. [Google Scholar] [CrossRef] [Green Version]















| Compound | COF-S7 | COF-S12 |
|---|---|---|
| Experimental surface area m2/g Theoretical pore opening | 3.44 | 20.73 |
| Large/Å | 14.02 | |
| Small/Å | 14.00 | 23.31 |
| Experimental pore opening (4VA/BET) | ||
| Large/Å | ||
| Small/Å | ||
| Experimental pore opening (4VA/BET) | ||
| Adsorption | 3.05 | 0.60 |
| Desorption | 160.65 | 71.69 |
| Average pore width (4V/A) | ||
| Adsorption/Å | 51.53 | 50.90 |
| Desorption/Å | 51.43 | 50.74 |
| Adsorbents | COF-S7 | COF-S12 |
|---|---|---|
| Experimental (qmax) | 35 | 42 |
| Calculated (qmax) | 11.710 | 24.600 |
| Langmuir KL (L/mg) | −0.474 | −0.154 |
| R2 | 0.788 | 0.811 |
| Freundlich n | −0.970 | −0.391 |
| 1/n | −1.030 | −2.556 |
| KF (mg/g) (1/mg) 1/n | 6.642 | 2.259 |
| R2 | 0.941 | 0.947 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, S.; Abdullah, C.A.C.; Tahir, M.I.M.; Abdul Rahman, M.B. Adsorptive Removal of Naproxen from Water Using Polyhedral Oligomeric Silesquioxane (POSS) Covalent Organic Frameworks (COFs). Nanomaterials 2022, 12, 2491. https://doi.org/10.3390/nano12142491
Bala S, Abdullah CAC, Tahir MIM, Abdul Rahman MB. Adsorptive Removal of Naproxen from Water Using Polyhedral Oligomeric Silesquioxane (POSS) Covalent Organic Frameworks (COFs). Nanomaterials. 2022; 12(14):2491. https://doi.org/10.3390/nano12142491
Chicago/Turabian StyleBala, Suleiman, Che Azurahanim Che Abdullah, Mohamed Ibrahim Mohamed Tahir, and Mohd Basyaruddin Abdul Rahman. 2022. "Adsorptive Removal of Naproxen from Water Using Polyhedral Oligomeric Silesquioxane (POSS) Covalent Organic Frameworks (COFs)" Nanomaterials 12, no. 14: 2491. https://doi.org/10.3390/nano12142491