Preliminary Evaluation of Iron Oxide Nanoparticles Radiolabeled with 68Ga and 177Lu as Potential Theranostic Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MIONs
2.1.1. Synthesis of MA and MAPEG
2.1.2. Dynamic Light Scattering
2.2. Radiolabeling MIONs with 68Ga
2.3. Radiolabeling MIONs with 177Lu
2.4. In Vitro Stability Studies of [68Ga]Ga-MIONs and [177Lu]Lu-MIONs
2.5. Hemolysis Assay
2.6. Cell Cultures
2.7. MTT Toxicity Assay
2.8. Ex Vivo Biodistribution Studies of the Radiolabeled MIONs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of MIONs
3.2. Radiolabeling of MIONs with 68Ga
3.3. In Vitro Stability of [68Ga]Ga-MIONs
3.4. Radiolabeling of MIONs with 177Lu
3.5. In Vitro Stability of [177Lu]Lu-MIONs
3.6. Hemolysis Assay
3.7. In Vitro Toxicity of MIONs
3.7.1. Cytotoxicity of MA and MAPEG


3.7.2. Cytotoxicity of [177Lu]Lu-MAPEG

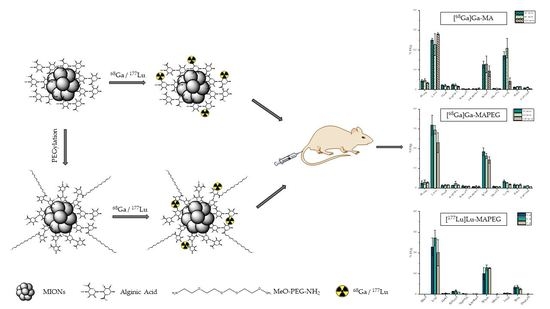
3.8. Ex Vivo Biodistribution Studies of [68Ga]Ga-MIONs in Normal Mice
3.9. Ex Vivo Biodistribution Studies of [177Lu]Lu-MAPEG in Normal Mice
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Same, S.; Aghanejad, A.; Akbari Nakhjavani, S.; Barar, J.; Omidi, Y. Radiolabeled Theranostics: Magnetic and Gold Nanoparticles. Bioimpacts 2016, 6, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoudeh, M.; Kamleh, M.A.; Diab, R.; Fessi, H. Radionuclides Delivery Systems for Nuclear Imaging and Radiotherapy of Cancer. Adv. Drug Deliv. Rev. 2008, 60, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Bauer, L.; Hoimes, C.; Ghaghada, K.B.; Karathanasis, E. Targeted Nanotechnology for Cancer Imaging. Adv. Drug Deliv. Rev. 2014, 76, 79–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Seniwal, B.; Thipe, V.C.; Singh, S.; Fonseca, T.C.F.; Freitas de Freitas, L. Recent Advances in Brachytherapy Using Radioactive Nanoparticles: An Alternative to Seed-Based Brachytherapy. Front. Oncol. 2021, 11, 4830. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef] [Green Version]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zhao, J.; Wen, X.; Wu, C.; Huang, Q.; Guan, F.; Wu, R.; Liang, D.; Li, C. Chemoradiation Therapy Using Cyclopamine-Loaded Liquid–Lipid Nanoparticles and Lutetium-177-Labeled Core-Crosslinked Polymeric Micelles. J. Control. Release 2015, 202, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-Gutiérrez, M.; Ocampo-García, B.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Trujillo-Benítez, D.; Hernández-Jiménez, T.; Ramírez-Nava, G.; Hernández-Ramírez, R.; Santos-Cuevas, C.; Ferro-Flores, G. Targeted Endoradiotherapy with Lu2O3-IPSMA/-IFAP Nanoparticles Activated by Neutron Irradiation: Preclinical Evaluation and First Patient Image. Pharmaceutics 2022, 14, 720. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular Imaging and Therapy of Cancer with Radiolabeled Nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-García, B.E.; de Ramírez, F.M. Radiolabeled Nanoparticles for Molecular Imaging; IntechOpen: Vienna, Austria, 2012; ISBN 978-953-51-0359-2. [Google Scholar]
- Salvanou, E.A.; Bouziotis, P.; Tsoukalas, C. Radiolabeled Nanoparticles in Nuclear Oncology. Adv. Nano Res. 2018, 1, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouziotis, P.; Psimadas, D.; Tsotakos, T.; Stamopoulos, D.; Tsoukalas, C. Radiolabeled Iron Oxide Nanoparticles as Dual-Modality SPECT/MRI and PET/MRI Agents. Curr. Top. Med. Chem. 2013, 12, 2694–2702. [Google Scholar] [CrossRef]
- Trujillo-Benítez, D.; Ferro-Flores, G.; Morales-Avila, E.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M.; Azorín-Vega, E. Synthesis and Biochemical Evaluation of Samarium-153 Oxide Nanoparticles Functionalized with IPSMA-Bombesin Heterodimeric Peptide. J. Biomed. Nanotechnol. 2020, 16, 689–701. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Wall, J.S.; Rondinone, A.J.; Kennel, S.J.; Mirzadeh, S.; Robertson, J.D. Gold Coated Lanthanide Phosphate Nanoparticles for Targeted Alpha Generator Radiotherapy. PLoS ONE 2013, 8, e54531. [Google Scholar] [CrossRef] [Green Version]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive Gold Nanoparticles in Cancer Therapy: Therapeutic Efficacy Studies of GA-198AuNP Nanoconstruct in Prostate Tumor–Bearing Mice. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 201–209. [Google Scholar] [CrossRef]
- Koziorowski, J.; Stanciu, A.; Gomez-Vallejo, V.; Llop, J. Radiolabeled Nanoparticles for Cancer Diagnosis and Therapy. Anti-Cancer Agents Med. Chem. 2017, 17, 333–354. [Google Scholar] [CrossRef]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, R.; Goel, S.; Dash, A.; Cai, W. Radiolabeled Inorganic Nanoparticles for Positron Emission Tomography Imaging of Cancer: An Overview. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, C.; Psimadas, D.; Kastis, G.A.; Koutoulidis, V.; Harris, A.L.; Paravatou-Petsotas, M.; Karageorgou, M.; Furenlid, L.R.; Moulopoulos, L.A.; Stamopoulos, D.; et al. A Novel Metal-Based Imaging Probe for Targeted Dual-Modality SPECT/MR Imaging of Angiogenesis. Front. Chem. 2018, 6, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madru, R.; Kjellman, P.; Olsson, F.; Wingårdh, K.; Ingvar, C.; Ståhlberg, F.; Olsrud, J.; Lätt, J.; Fredriksson, S.; Knutsson, L.; et al. 99mTc-Labeled Superparamagnetic Iron Oxide Nanoparticles for Multimodality SPECT/MRI of Sentinel Lymph Nodes. J. Nucl. Med. 2012, 53, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-Y.; Li, Z.; Chen, K.; Hsu, A.R.; Xu, C.; Xie, J.; Sun, S.; Chen, X. PET/MRI Dual-Modality Tumor Imaging Using Arginine-Glycine-Aspartic (RGD)-Conjugated Radiolabeled Iron Oxide Nanoparticles. J. Nucl. Med. 2008, 49, 1371–1379. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Molecular Imaging with 68Ga Radio-Nanomaterials: Shedding Light on Nanoparticles. Appl. Sci. 2018, 8, 1098. [Google Scholar] [CrossRef] [Green Version]
- Karageorgou, M.-A.; Vranješ-Djurić, S.; Radović, M.; Lyberopoulou, A.; Antić, B.; Rouchota, M.; Gazouli, M.; Loudos, G.; Xanthopoulos, S.; Sideratou, Z.; et al. Gallium-68 Labeled Iron Oxide Nanoparticles Coated with 2,3-Dicarboxypropane-1,1-Diphosphonic Acid as a Potential PET/MR Imaging Agent: A Proof-of-Concept Study. Contrast Media Mol. Imaging 2017, 2017, 6951240. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Chen, X. Multimodality Molecular Imaging of Tumor Angiogenesis. J. Nucl. Med. 2008, 49 (Suppl. S2), 113S–128S. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ellison, P.A.; Lewis, C.M.; Hong, H.; Zhang, Y.; Shi, S.; Hernandez, R.; Meyerand, M.E.; Barnhart, T.E.; Cai, W. Chelator-Free Synthesis of a Dual-Modality PET/MRI Agent. Angew. Chem. Int. Ed. Engl. 2013, 52, 13319–13323. [Google Scholar] [CrossRef]
- Pichler, B.J.; Wehrl, H.F.; Kolb, A.; Judenhofer, M.S. Positron Emission Tomography/Magnetic Resonance Imaging: The next Generation of Multimodality Imaging? Semin. Nucl. Med. 2008, 38, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Giammarile, F.; Castellucci, P.; Dierckx, R.; Estrada Lobato, E.; Farsad, M.; Hustinx, R.; Jalilian, A.; Pellet, O.; Rossi, S.; Paez, D. Non-FDG PET/CT in Diagnostic Oncology: A Pictorial Review. Eur. J. Hybrid Imaging 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S. Translational Multimodality Neuroimaging. Curr. Drug Targets 2017, 18, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled Peptides: Valuable Tools for the Detection and Treatment of Cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassis, A.I. Therapeutic Radionuclides: Biophysical and Radiobiologic Principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radović, M.; Calatayud, M.P.; Goya, G.F.; Ibarra, M.R.; Antić, B.; Spasojević, V.; Nikolić, N.; Janković, D.; Mirković, M.; Vranješ-Đurić, S. Preparation and in Vivo Evaluation of Multifunctional 90Y-Labeled Magnetic Nanoparticles Designed for Cancer Therapy. J. Biomed. Mater. Res. Part A 2015, 103, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ognjanović, M.; Radović, M.; Mirković, M.; Prijović, Ž.; del Puerto Morales, M.; Čeh, M.; Vranješ-Đurić, S.; Antić, B. 99mTc-, 90Y-, and 177Lu-Labeled Iron Oxide Nanoflowers Designed for Potential Use in Dual Magnetic Hyperthermia/Radionuclide Cancer Therapy and Diagnosis. ACS Appl. Mater. Interfaces 2019, 11, 41109–41117. [Google Scholar] [CrossRef] [PubMed]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Żuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.-A.; Bouziotis, P.; Bilewicz, A. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef] [Green Version]
- Gawęda, W.; Pruszyński, M.; Cędrowska, E.; Rodak, M.; Majkowska-Pilip, A.; Gaweł, D.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials 2020, 10, 2067. [Google Scholar] [CrossRef]
- Gawęda, W.; Osial, M.; Żuk, M.; Pękała, M.; Bilewicz, A.; Krysinski, P. Lanthanide-Doped SPIONs Bioconjugation with Trastuzumab for Potential Multimodal Anticancer Activity and Magnetic Hyperthermia. Nanomaterials 2020, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of 177Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef] [Green Version]
- Zoppellaro, G.; Kolokithas-Ntoukas, A.; Polakova, K.; Tucek, J.; Zboril, R.; Loudos, G.; Fragogeorgi, E.; Diwoky, C.; Tomankova, K.; Avgoustakis, K.; et al. Theranostics of Epitaxially Condensed Colloidal Nanocrystal Clusters, through a Soft Biomineralization Route. Chem. Mater. 2014, 26, 2062–2074. [Google Scholar] [CrossRef]
- Sarigiannis, Y.; Kolokithas-Ntoukas, A.; Beziere, N.; Zbořil, R.; Papadimitriou, E.; Avgoustakis, K.; Lamprou, M.; Medrikova, Z.; Rousalis, E.; Ntziachristos, V.; et al. Synthesis and Evaluation of Condensed Magnetic Nanocrystal Clusters with in Vivo Multispectral Optoacoustic Tomography for Tumour Targeting. Biomaterials 2016, 91, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Kolokithas-Ntoukas, A.; Fytas, C.; Avgoustakis, K. Folic Acid-Functionalized, Condensed Magnetic Nanoparticles for Targeted Delivery of Doxorubicin to Tumor Cancer Cells Overexpressing the Folate Receptor. ACS Omega 2019, 4, 22214–22227. [Google Scholar] [CrossRef]
- Papadopoulou, S.; Kolokithas-Ntoukas, A.; Salvanou, E.-A.; Gaitanis, A.; Xanthopoulos, S.; Avgoustakis, K.; Gazouli, M.; Paravatou-Petsotas, M.; Tsoukalas, C.; Bakandritsos, A.; et al. Chelator-Free/Chelator-Mediated Radiolabeling of Colloidally Stabilized Iron Oxide Nanoparticles for Biomedical Imaging. Nanomaterials 2021, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Kolokithas-Ntoukas, A.; Bakandritsos, A.; Belza, J.; Kesa, P.; Herynek, V.; Pankrac, J.; Angelopoulou, A.; Malina, O.; Avgoustakis, K.; Georgakilas, V.; et al. Condensed Clustered Iron Oxides for Ultrahigh Photothermal Conversion and In Vivo Multimodal Imaging. ACS Appl. Mater. Interfaces 2021, 13, 29247–29256. [Google Scholar] [CrossRef]
- Coral, D.F.; Soto, P.A.; Blank, V.; Veiga, A.; Spinelli, E.; Gonzalez, S.; Saracco, G.P.; Bab, M.A.; Muraca, D.; Setton-Avruj, P.C.; et al. Nanoclusters of Crystallographically Aligned Nanoparticles for Magnetic Thermotherapy: Aqueous Ferrofluid, Agarose Phantoms and Ex Vivo Melanoma Tumour Assessment. Nanoscale 2018, 10, 21262–21274. [Google Scholar] [CrossRef] [Green Version]
- Kostopoulou, A.; Velu, S.K.P.; Thangavel, K.; Orsini, F.; Brintakis, K.; Psycharakis, S.; Ranella, A.; Bordonali, L.; Lappas, A.; Lascialfari, A. Colloidal Assemblies of Oriented Maghemite Nanocrystals and Their NMR Relaxometric Properties. Dalton Trans. 2014, 43, 8395–8404. [Google Scholar] [CrossRef] [Green Version]
- Sakellari, D.; Brintakis, K.; Kostopoulou, A.; Myrovali, E.; Simeonidis, K.; Lappas, A.; Angelakeris, M. Ferrimagnetic Nanocrystal Assemblies as Versatile Magnetic Particle Hyperthermia Mediators. Mater. Sci. Eng. C 2016, 58, 187–193. [Google Scholar] [CrossRef]
- Shaw, S.K.; Kailashiya, J.; Gangwar, A.; Alla, S.K.; Gupta, S.K.; Prajapat, C.L.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N.K. γ-Fe2O3 Nanoflowers as Efficient Magnetic Hyperthermia and Photothermal Agent. Appl. Surf. Sci. 2021, 560, 150025. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, C.; Chen, D.-X.; Gu, H. Magnetite Nanocrystal Clusters with Ultra-High Sensitivity in Magnetic Resonance Imaging. ChemPhysChem 2012, 13, 336–341. [Google Scholar] [CrossRef]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rosch, F. Processing of Generator-Produced 68Ga for Medical Application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.P.; Baghdadi, N.; Clemente, G.S.; Camus, N.; Guillou, A.; Kownacka, A.E.; Domarkas, J.; Halime, Z.; Tripier, R.; Archibald, S.J. Final Step Gallium-68 Radiolabelling of Silica-Coated Iron Oxide Nanorods as Potential PET/MR Multimodal Imaging Agents. Faraday Discuss. 2014, 175, 59–71. [Google Scholar] [CrossRef] [PubMed]
- de la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. Mutual Interaction of Red Blood Cells Influenced by Nanoparticles. Sci. Rep. 2019, 9, 5147. [Google Scholar] [CrossRef]
- Goodhead, L.K.; MacMillan, F.M. Measuring Osmosis and Hemolysis of Red Blood Cells. Adv. Physiol. Educ. 2017, 41, 298–305. [Google Scholar] [CrossRef]
- Bartholomä, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and Gallium Derived Radiopharmaceuticals: Comparing and Contrasting the Chemistry of Two Important Radiometals for the Molecular Imaging Era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef]
- Rey-Castro, C.; Herrero, R.; Sastre de Vicente, M.E. Gibbs–Donnan and Specific-Ion Interaction Theory Descriptions of the Effect of Ionic Strength on Proton Dissociation of Alginic Acid. J. Electroanal. Chem. 2004, 564, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Luna-Gutiérrez, M.; Ferro-Flores, G.; Ocampo-García, B.E.; Santos- Cuevas, C.L.; Jiménez-Mancilla, N.; De León-Rodríguez, L.M.; Azorín-Vega, E.; Isaac- Olivé, K. A Therapeutic System of 177Lu-Labeled Gold Nanoparticles-RGD Internalized in Breast Cancer Cells. J. Mex. Chem. Soc. 2013, 57, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Leonte, R.A.; Chilug, L.E.; Șerban, R.; Mustăciosu, C.; Raicu, A.; Manda, G.; Niculae, D. Preparation and Preliminary Evaluation of Neurotensin Radiolabelled with 68Ga and 177Lu as Potential Theranostic Agent for Colon Cancer. Pharmaceutics 2021, 13, 506. [Google Scholar] [CrossRef]
- Theodosiou, M.; Sakellis, E.; Boukos, N.; Kusigerski, V.; Kalska-Szostko, B.; Efthimiadou, E. Iron Oxide Nanoflowers Encapsulated in Thermosensitive Fluorescent Liposomes for Hyperthermia Treatment of Lung Adenocarcinoma. Sci. Rep. 2022, 12, 8697. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Chiotellis, A.; Salvanou, E.-A.; Makrypidi, K.; Tsoukalas, C.; Kapiris, F.; Paravatou-Petsotas, M.; Papadopoulos, M.; Pirmettis, I.C.; Koźmiński, P.; et al. Synthesis and In Vitro Evaluation of Gold Nanoparticles Functionalized with Thiol Ligands for Robust Radiolabeling with 99mTc. Nanomaterials 2021, 11, 2406. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Dannenfelser, R.-M. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-García, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in Vitro Evaluation of Radiolabeled HA-PLGA Nanoparticles as Novel MTX Delivery System for Local Treatment of Rheumatoid Arthritis. Mater. Sci. Eng. C 2019, 103, 109766. [Google Scholar] [CrossRef]
- Müller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-Targeted Radionuclide Therapy of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1919–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Lahooti, A.; Sarkar, S.; Saligheh Rad, H.; Gholami, A.; Nosrati, S.; Muller, R.N.; Laurent, S.; Grüttner, C.; Geramifar, P.; Yousefnia, H.; et al. PEGylated Superparamagnetic Iron Oxide Nanoparticles Labeled with 68Ga as a PET/MRI Contrast Agent: A Biodistribution Study. J. Radioanal. Nucl. Chem. 2017, 311, 769–774. [Google Scholar] [CrossRef]
- Yook, S.; Lu, Y.; Jeong, J.J.; Cai, Z.; Tong, L.; Alwarda, R.; Pignol, J.-P.; Winnik, M.A.; Reilly, R.M. Stability and Biodistribution of Thiol-Functionalized and 177Lu-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules 2016, 17, 1292–1302. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Liolios, C.; Xanthopoulos, S.; Paravatou-Petsotas, M.; Tsoukalas, C.; Avgoustakis, K.; Bouziotis, P. Preliminary Evaluation of Iron Oxide Nanoparticles Radiolabeled with 68Ga and 177Lu as Potential Theranostic Agents. Nanomaterials 2022, 12, 2490. https://doi.org/10.3390/nano12142490
Salvanou E-A, Kolokithas-Ntoukas A, Liolios C, Xanthopoulos S, Paravatou-Petsotas M, Tsoukalas C, Avgoustakis K, Bouziotis P. Preliminary Evaluation of Iron Oxide Nanoparticles Radiolabeled with 68Ga and 177Lu as Potential Theranostic Agents. Nanomaterials. 2022; 12(14):2490. https://doi.org/10.3390/nano12142490
Chicago/Turabian StyleSalvanou, Evangelia-Alexandra, Argiris Kolokithas-Ntoukas, Christos Liolios, Stavros Xanthopoulos, Maria Paravatou-Petsotas, Charalampos Tsoukalas, Konstantinos Avgoustakis, and Penelope Bouziotis. 2022. "Preliminary Evaluation of Iron Oxide Nanoparticles Radiolabeled with 68Ga and 177Lu as Potential Theranostic Agents" Nanomaterials 12, no. 14: 2490. https://doi.org/10.3390/nano12142490