Biomechanical Analysis of Axial Gradient Porous Dental Implants: A Finite Element Analysis
Abstract
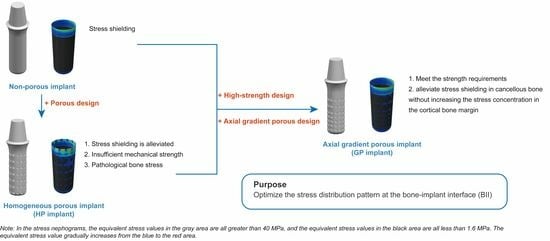
:1. Introduction
2. Materials and Methods
2.1. Construction of a Three-Dimensional (3D) Geometric Model
2.1.1. Construction of the Mandibular Model
2.1.2. Construction of a Standard Implant Model for the Control Group
2.1.3. Construction of homogeneous porous (HP) implant models
2.1.4. Construction of High-Strength Designed Implant Models
2.1.5. Construction of the Axial GP Implant Model
2.1.6. Assembly of FEA Models
2.2. Pre-Processing of FEA
2.3. Analytical Methods and Indicators
3. Results
3.1. Stress Distribution of HP Implants
3.1.1. Axial Variation Trend of Equivalent Stress
3.1.2. Interval Distribution of Equivalent Stress
3.1.3. Peak Equivalent Stress
3.2. Stress Distribution of High-Strength Designed Implants
3.2.1. Peak Equivalent Stress in Implants
3.2.2. Interval Distribution of Equivalent Stress
3.3. Stress Distribution of GP Implants
3.3.1. Peak Equivalent Stress
3.3.2. Axial Variation Trend of Equivalent Stress
3.3.3. Interval Distribution of Equivalent Stress
4. Discussion
4.1. Stress Distribution Regularity and Ideal Stress Range
4.2. Effect of the GP Implant on Stress Distribution
4.3. The Clinical Implications of the Findings
4.4. Study Limitations
5. Conclusions
- When dental implants are subjected to masticatory pressure, the stress concentration is prominent on the neck and apex areas of the BII.
- The GP implants (as shown in Figure A2) meet the ideal strength requirements, making them suitable for implant applications.
- GP implants can alleviate the stress shielding in cancellous bone without increasing the stress concentration in the cortical bone margin, thus achieving the purpose of optimizing the stress distribution pattern at the BII.
- Under the premise of ensuring the quality of the preparation process, T3/5 (for which, as shown in Figure A2, the porosity of the crown direction region is 55% and the porosity of the root direction region is 75%) is the best GP implant design.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Kang, X.; Li, Y.; Wang, Y.; Zhang, Y.; Yu, D.; Peng, Y. Relationships of Stresses on Alveolar Bone and Abutment of Dental Implant from Various Bite Forces by Three-Dimensional Finite Element Analysis. BioMed Res. Int. 2020, 2020, 7539628. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; Verri, F.R.; Noritomi, P.Y.; Kemmoku, D.T.; Batista, V.E.S.; Cruz, R.S.; de Luna Gomes, J.M.; Pellizzer, E.P. Effect of bone quality and bone loss level around internal and external connection implants: A finite element analysis study. J. Prosthet. Dent. 2021, 125, 137.e1–137.e10. [Google Scholar] [CrossRef] [PubMed]
- Yenigun, S.; Ercal, P.; Ozden-Yenigun, E.; Katiboglu, A.B. Influence of Abutment Design on Stress Distribution in Narrow Implants with Marginal Bone Loss: A Finite Element Analysis. Int. J. Oral Maxillofac. Implant. 2021, 36, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Mito, T.; Yoda, N.; Sato, E.; Shigemitsu, R.; Han, J.M.; Sasaki, K. Effect of peri-implant bone resorption on mechanical stress in the implant body: In vivo measured load-based finite element analysis. J. Oral Rehabil. 2020, 47, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Velasco, A.B.; Ríos-Santos, J.V.; Lasheras, F.S.; Lemos, B.F.; Gil, F.J.; Carvalho, A.; Herrero-Climent, M. Effect of Different Implant Designs on Strain and Stress Distribution under Non-Axial Loading: A Three-Dimensional Finite Element Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4738. [Google Scholar] [CrossRef]
- Sivrikaya, E.C.; Omezli, M.M. The Effect of Tapered and Cylindrical Implants on Stress Distribution in Different Bone Qualities: A Finite Element Analysis. Int. J. Oral Maxillofac. Implant. 2019, 34, e99–e105. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef]
- Kumar, G.; Tison, C.K.; Chatterjee, K.; Pine, P.S.; McDaniel, J.H.; Salit, M.L.; Young, M.F.; Simon, C.G., Jr. The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials 2011, 32, 9188–9196. [Google Scholar] [CrossRef]
- Faverani, L.P.; Barão, V.A.; Ramalho-Ferreira, G.; Delben, J.A.; Ferreira, M.B.; Júnior, I.R.G.; Assunção, W.G. The influence of bone quality on the biomechanical behavior of full-arch implant-supported fixed prostheses. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 164–170. [Google Scholar] [CrossRef]
- Mishchenko, O.; Ovchynnykov, O.; Kapustian, O.; Pogorielov, M. New Zr-Ti-Nb Alloy for Medical Application: Development, Chemical and Mechanical Properties, and Biocompatibility. Materials 2020, 13, 1306. [Google Scholar] [CrossRef]
- Heary, R.F.; Parvathreddy, N.; Sampath, S.; Agarwal, N. Elastic modulus in the selection of interbody implants. J. Spine Surg. 2017, 3, 163–167. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Engineering. C Mater. Biol. Appl. 2019, 104, 109908. [Google Scholar] [CrossRef] [PubMed]
- Sadati, M.; Ghofrani, S.; Mehrizi, A.A. Investigation of porous cells interface on elastic property of orthopedic implants: Numerical and experimental studies. J. Mech. Behav. Biomed. Mater. 2021, 120, 104595. [Google Scholar] [CrossRef]
- Wang, H.; Wan, Y.; Li, Q.; Xia, Y.; Liu, X.; Liu, Z.; Li, X. Porous fusion cage design via integrated global-local topology optimization and biomechanical analysis of performance. J. Mech. Behav. Biomed. Mater. 2020, 112, 103982. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.M.; Cheng, K.J.; Liu, Y.F.; Nizza, M.; Baur, D.A.; Jiang, X.F.; Dong, X.T. Biomechanical and Mechanostat analysis of a titanium layered porous implant for mandibular reconstruction: The effect of the topology optimization design. Mater. Sci. Eng. C 2021, 124, 112056. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Zhou, Q.; Gong, Y.; Li, R.; Li, C.; KlÃ, F.; Freund, S.; Wu, X.; Sun, Y.; et al. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implants: Fabrication, biocompatibility analysis and photoelastic study. Sci. Rep. 2017, 7, 45360. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Tang, Y.; Yang, G.; Wu, X.; Lin, S.; Liu, X.; Cao, H.; Jiang, X. Preservation of alveolar ridge height through mechanical memory: A novel dental implant design. Bioact. Mater. 2021, 6, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Gao, H.; Liu, T.; Huang, Y.; Wang, C. Effects of immediately static loading on osteointegration and osteogenesis around 3D-printed porous implant: A histological and biomechanical study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110406. [Google Scholar] [CrossRef]
- Yang, L.; Yan, C.; Fan, H.; Li, Z.; Cai, C.; Chen, P.; Shi, Y.; Yang, S. Investigation on the orientation dependence of elastic response in Gyroid cellular structures. J. Mech. Behav. Biomed. Mater. 2019, 90, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Bataineh, K.; Al Janaideh, M. Effect of different biocompatible implant materials on the mechanical stability of dental implants under excessive oblique load. Clin. Implant. Dent. Relat. Res. 2019, 21, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Sarna-Bos, K.; Skic, K.; Sobieszczanski, J.; Boguta, P.; Chalas, R. Contemporary Approach to the Porosity of Dental Materials and Methods of Its Measurement. Int. J. Mol. Sci. 2021, 22, 8903. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Cheong, V.S.; Fromme, P.; Coathup, M.J.; Mumith, A.; Blunn, G.W. Partial Bone Formation in Additive Manufactured Porous Implants Reduces Predicted Stress and Danger of Fatigue Failure. Ann. Biomed. Eng. 2020, 48, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, W.; Chen, M.; Chen, D.; Sun, G.; Zhang, C.; Pan, Y.; Lu, J.; Guo, E.; Lu, X. Structural Design and Finite Element Simulation Analysis of Grade 3 Graded Porous Titanium Implant. Int. J. Mol. Sci. 2022, 23, 10090. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wu, L.; Lei, H.; Zhou, C.; Fan, H.; Li, Z.; Zhang, B.; Sun, H.; Gui, X.; Jiang, Q.; et al. Fabrication of customized Ti6AI4V heterogeneous scaffolds with selective laser melting: Optimization of the architecture for orthopedic implant applications. Acta Biomater. 2021, 126, 485–495. [Google Scholar] [CrossRef]
- Bahrami, B.; Shahrbaf, S.; Mirzakouchaki, B.; Ghalichi, F.; Ashtiani, M.; Martin, N. Effect of surface treatment on stress distribution in immediately loaded dental implants—A 3D finite element analysis. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, e89–e97. [Google Scholar] [CrossRef]
- Song, K.; Wang, Z.; Lan, J.; Ma, S. Porous structure design and mechanical behavior analysis based on TPMS for customized root analogue implant. J. Mech. Behav. Biomed. Mater. 2021, 115, 104222. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Apicella, A.; Mu, Z.; Yu, T.; Huang, Y.; Wang, C. Effect of Porous Microstructures on the Biomechanical Characteristics of a Root Analogue Implant: An Animal Study and a Finite Element Analysis. ACS Biomater. Sci. Eng. 2020, 6, 6356–6367. [Google Scholar] [CrossRef]
- Cruz, R.S.; Fernandes, E.O.H.F.; Lemos, C.A.A.; de Souza Batista, V.E.; da Silva, R.C.; Verri, F.R. Biomechanical influence of narrow-diameter implants placed at the crestal and subcrestal level in the maxillary anterior region. A 3D finite element analysis. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/36799260/ (accessed on 1 October 2023). [CrossRef] [PubMed]
- Uto, Y.; Kuroshima, S.; Nakano, T.; Ishimoto, T.; Inaba, N.; Uchida, Y.; Sawase, T. Effects of mechanical repetitive load on bone quality around implants in rat maxillae. PLoS ONE 2017, 12, e0189893. [Google Scholar] [CrossRef] [PubMed]
- Samiezadeh, S.; Bougherara, H.; Abolghasemian, M.; D’lima, D.; Backstein, D. Rotating hinge knee causes lower bone-implant interface stress compared to constrained condylar knee replacement. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Brune, A.; Stiesch, M.; Eisenburger, M.; Greuling, A. The effect of different occlusal contact situations on peri-implant bone stress—A contact finite element analysis of indirect axial loading. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Suedam, V.; Neto, R.T.M.; Sousa, E.A.; Rubo, J.H. Effect of cantilever length and alloy framework on the stress distribution in peri-implant area of cantilevered implant-supported fixed partial dentures. J. Appl. Oral Sci. Rev. FOB 2016, 24, 114–120. [Google Scholar] [CrossRef]
- Santos, M.B.D.; Da Silva Neto, J.P.; Consani, R.L.; Mesquita, M.F. Three-dimensional finite element analysis of stress distribution in peri-implant bone with relined dentures and different heights of healing caps. J. Oral Rehabil. 2011, 38, 691–696. [Google Scholar] [CrossRef]
- Yamanishi, Y.; Yamaguchi, S.; Imazato, S.; Nakano, T.; Yatani, H. Effects of the implant design on peri-implant bone stress and abutment micromovement: Three-dimensional finite element analysis of original computer-aided design models. J. Periodontol. 2014, 85, e333–e338. [Google Scholar] [CrossRef]
- Mao, C.; Yu, W.; Jin, M.; Wang, Y.; Shang, X.; Lin, L.; Zeng, X.; Wang, L.; Lu, E. Mechanobiologically optimized Ti–35Nb–2Ta–3Zr improves load transduction and enhances bone remodeling in tilted dental implant therapy. Bioact. Mater. 2022, 16, 15–26. [Google Scholar] [CrossRef]
- Ouldyerou, A.; Mehboob, H.; Merdji, A.; Aminallah, L.; Mehboob, A.; Mukdadi, O.M. Biomechanical analysis of printable functionally graded material (FGM) dental implants for different bone densities. Comput. Biol. Med. 2022, 150, 106111. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar]
- Geng, J.-P.; Tan, K.B.C.; Liu, G.-R. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, B.; Baptista, A.A.; Patoor, E.; Bravetti, P.; Eberhardt, A.; Laheurte, P. Interaction of bone-dental implant with new ultra low modulus alloy using a numerical approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 151–160. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Hillmyer, M.A.; Francis, L.F. Processing and properties of porous poly(L-lactide)/bioactive glass composites. Biomaterials 2004, 25, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: London, UK, 1997; pp. 85–114. [Google Scholar]
- Misch, C.E.; Bidez, M.W.; Sharawy, M. A Bioengineered Implant for a Predetermined Bone Cellular Response to Loading Forces. A Literature Review and Case Report. J. Periodontol. 2001, 72, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, M.; Cugnoni, J.; Botsis, J.; Ammann, P.; Wiskott, A. Peri-implant bone adaptations to overloading in rat tibiae: Experimental investigations and numerical predictions. Clin. Oral Implant. Res. 2016, 27, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, Q.; Li, W.; Zhou, S.; Swain, M.V. Design optimization of functionally graded dental implant for bone remodeling. Compos. Part B Eng. 2009, 40, 668–675. [Google Scholar] [CrossRef]
- Trueba, P.; Navarro, C.; Rodríguez-Ortiz, J.E.A.; Beltr, A.M.; García-García, F.J.; Torres, Y. Fabrication and characterization of superficially modified porous dental implants. Surf. Coat. Technol. 2021, 408, 126796. [Google Scholar] [CrossRef]
- Kamboj, N.; Kazantseva, J.; Rahmani, R.; Rodriguez, M.A.; Hussainova, I. Selective laser sintered bio-inspired silicon-wollastonite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111223. [Google Scholar] [CrossRef]
- Arindam, C.; Pallab, D.; Santanu, M.; Chandra, M.S.; Amit, R. Finite element and experimental analysis to select patient’s bone condition specific porous dental implant, fabricated using additive manufacturing. Comput. Biol. Med. 2020, 124, 103839. [Google Scholar]
- Zhang, C.; Zeng, C.; Wang, Z.; Zeng, T.; Wang, Y. Optimization of stress distribution of bone-implant interface (BII). Biomater. Adv. 2023, 147, 213342. [Google Scholar] [CrossRef]











| T1/2 | T1/3 | T1/4 | T1/5 | T2/3 | T2/4 | T2/5 | T3/4 | T3/5 | T4/5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Porosity in the crown direction (%) | 35 | 35 | 35 | 35 | 45 | 45 | 45 | 55 | 55 | 65 |
| Porosity in the root direction (%) | 45 | 55 | 65 | 75 | 55 | 65 | 75 | 65 | 75 | 75 |
| Components | Number of Elements | Number of Nodes | Components | Number of Elements | Number of Nodes |
|---|---|---|---|---|---|
| Cancellous bone | 140,067 | 29,474 | T1/2 | 112,942 | 25,931 |
| Cortical bone | 102,561 | 24,164 | T1/3 | 113,687 | 26,382 |
| T0 | 38,715 | 8742 | T1/4 | 119,426 | 28,469 |
| T1 | 112,898 | 27,734 | T1/5 | 116,513 | 28,689 |
| T2 | 131,591 | 34,511 | T2/3 | 118,881 | 28,600 |
| T3 | 117,549 | 32,813 | T2/4 | 122,488 | 30,220 |
| T4 | 114,341 | 34,422 | T2/5 | 120,861 | 30,616 |
| T5 | 181,394 | 57,613 | T3/4 | 116,900 | 29,490 |
| T3/5 | 114,689 | 29,786 | |||
| T4/5 | 120,602 | 31,660 |
| Model | S < 1.6 MPa | 1.6 MPa ≤ S < 40 MPa | S ≥ 40 MPa | |||
|---|---|---|---|---|---|---|
| Oblique Load (%) | Vertical Load (%) | Oblique Load (%) | Vertical Load (%) | Oblique Load (%) | Vertical Load (%) | |
| T0 | 79.65 | 79.03 | 20.30 | 20.97 | 0.05 | 0 |
| T1 | 66.74 | 73.42 | 32.67 | 26.48 | 0.59 | 0.11 |
| T2 | 66.78 | 74.25 | 32.65 | 25.68 | 0.57 | 0.07 |
| T3 | 61.13 | 70.32 | 38.22 | 29.58 | 0.66 | 0.10 |
| T4 | 57.63 | 68.32 | 41.18 | 31.1 | 1.19 | 0.49 |
| T5 | 53.71 | 65.28 | 44.80 | 33.77 | 1.50 | 0.94 |
| Model | S < 1.6 MPa | 1.6 MPa ≤ S < 40 MPa | S ≥ 40 MPa | |||
|---|---|---|---|---|---|---|
| Oblique Load (%) | Vertical Load (%) | Oblique Load (%) | Vertical Load (%) | Oblique Load (%) | Vertical Load (%) | |
| T3P | 59.65 | 66.12 | 39.83 | 33.83 | 0.52 | 0.06 |
| T3C | 67.89 | 73.29 | 31.86 | 26.71 | 0.25 | 0 |
| T3B | 71.26 | 75.41 | 28.48 | 24.59 | 0.25 | 0 |
| T3PC | 67.15 | 71.62 | 32.67 | 28.39 | 0.18 | 0 |
| T3PB | 70.29 | 74.56 | 29.55 | 25.44 | 0.16 | 0 |
| Load Type | Implant Type | SMax (MPa) | Mean | Range | Standard Deviation |
|---|---|---|---|---|---|
| 118.2 N oblique load | GP implants | 45.83, 46.04, 46.86, 47.51, 46.72, 47.32, 47.96, 48.10, 48.72, 50.34 | 47.54 | 4.51 | 1.268 |
| HP implants | 209.7, 194.4, 209.9, 358.0, 364.6 | 267.30 | 170.20 | 76.968 | |
| 120 N vertical load | GP implants | 19.53, 19.74, 19.97, 20.29, 19.81, 20.09, 20.36, 20.38, 20.77, 21.12 | 20.21 | 1.59 | 0.460 |
| HP implants | 82.88, 71.25, 74.07, 117.2, 125.8 | 94.24 | 54.55 | 22.749 |
| Model | S < 1.6 MPa | 1.6 MPa ≤ S < 40 MPa | S ≥ 40 MPa | |||
|---|---|---|---|---|---|---|
| Oblique Load (%) | Vertical Load (%) | Oblique Load (%) | Vertical Load (%) | Oblique Load (%) | Vertical Load (%) | |
| T0 | 79.65 | 79.03 | 20.30 | 20.97 | 0.05 | 0 |
| T1/2 | 71.50 | 74.70 | 28.37 | 25.30 | 0.13 | 0 |
| T1/3 | 69.68 | 73.44 | 30.18 | 26.56% | 0.13 | 0 |
| T1/4 | 66.11 | 70.80 | 33.75 | 29.20 | 0.14 | 0 |
| T1/5 | 62.06 | 68.31 | 37.77 | 31.69 | 0.17 | 0 |
| T2/3 | 66.79 | 70.01 | 33.08 | 29.99 | 0.13 | 0 |
| T2/4 | 62.00 | 66.30 | 37.84 | 33.70 | 0.16 | 0 |
| T2/5 | 60.53 | 66.36 | 39.29 | 33.64 | 0.18 | 0 |
| T3/4 | 64.42 | 69.23 | 35.40 | 30.77 | 0.18 | 0 |
| T3/5 | 59.47 | 65.43 | 40.34 | 34.57 | 0.18 | 0 |
| T4/5 | 59.05 | 65.60 | 40.72 | 34.40 | 0.23 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Y. Biomechanical Analysis of Axial Gradient Porous Dental Implants: A Finite Element Analysis. J. Funct. Biomater. 2023, 14, 557. https://doi.org/10.3390/jfb14120557
Zhang C, Wang Y. Biomechanical Analysis of Axial Gradient Porous Dental Implants: A Finite Element Analysis. Journal of Functional Biomaterials. 2023; 14(12):557. https://doi.org/10.3390/jfb14120557
Chicago/Turabian StyleZhang, Chunyu, and Yuehong Wang. 2023. "Biomechanical Analysis of Axial Gradient Porous Dental Implants: A Finite Element Analysis" Journal of Functional Biomaterials 14, no. 12: 557. https://doi.org/10.3390/jfb14120557