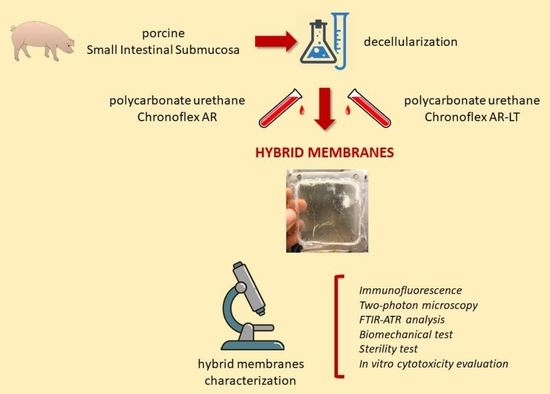
A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Porcine SIS Decellularization
2.2. Hybrid Membrane Fabrication
2.3. Immunofluorescence
2.4. Two-Photon Microscopy
2.5. FTIR Analysis
2.6. Mechanical Tests
2.7. Hybrid Membrane Sterilization
2.8. Sterility Evaluation
2.9. In Vitro Cytocompatibility Evaluation
2.10. DNA Quantification
2.11. Live/Dead Assays
2.12. WST Assay
3. Results
3.1. Morphology Evaluation of Hybrid Membranes
3.2. Polymers Penetration in Decellularized SIS
3.3. Biomechanical Characterization
3.4. Sterility Assessment
3.5. In Vitro Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Med. Lith. 2021, 57, 749. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Mirabelli, P.; Coppola, L.; Illiano, E.; Salvatore, M.; Franzese, M. New Roadmaps for Non-Muscle-Invasive Bladder Cancer With Unfavorable Prognosis. Front. Chem. 2020, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–176. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Nielsen, M.; Smith, A.; Nix, J.; Schultz, H.; Wallen, E.M. Fast Track Program in Patients Undergoing Radical Cystectomy: Results in 362 Consecutive Patients. J. Am. Coll. Surg. 2010, 210, 93–99. [Google Scholar] [CrossRef]
- Ramirez, J.A.; McIntosh, A.G.; Strehlow, R.; Lawrence, V.A.; Parekh, D.J.; Svatek, R.S. Definition, Incidence, Risk Factors, and Prevention of Paralytic Ileus Following Radical Cystectomy: A Systematic Review. Eur. Urol. 2013, 64, 588–597. [Google Scholar] [CrossRef]
- Bazargani, S.T.; Djaladat, H.; Ahmadi, H.; Miranda, G.; Cai, J.; Schuckman, A.K.; Daneshmand, S. Gastrointestinal Complications Following Radical Cystectomy Using Enhanced Recovery Protocol. Eur. Urol. Focus 2018, 4, 889–894. [Google Scholar] [CrossRef]
- Faba, O.R.; Moreno, R.P.; Malca, L.; Martínez, A.P.; Nervo, N.; Breda, A.; Esquinas, C.; Palou, J. Postoperative Management of Radical Cystectomy. Review of the Evidence on the Prevention and Treatment of Urological Complications. Actas Urol. Esp. Engl. Ed. 2018, 42, 143–151. [Google Scholar] [CrossRef]
- Shelbaia, A.; Salem, H.K.; Emran, A.; Raouf, M.A.; Rahman, S.A. Long Term Complications after Radical Cystoprostatectomy with Orthotopic Diversion in Male Patients: Preliminary Experience. Afr. J. Urol. 2013, 19, 89–93. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pokrywczynska, M.; Van Breda, S.V.; Kloskowski, T.; Drewa, T. Concise Review: Tissue Engineering of Urinary Bladder; We Still Have a Long Way to Go? Stem Cells Transl. Med. 2017, 6, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.G.; Knight, T.A. Design, Fabrication, and Preparation of Synthetic Scaffolds for Urologic Tissue Engineering. Methods Mol. Biol. Clifton NJ 2013, 1001, 167–177. [Google Scholar] [CrossRef]
- Knight, T.A.; Payne, R.G. Characterization of a PGA-Based Scaffold for Use in a Tissue-Engineered Neo-Urinary Conduit. Methods Mol. Biol. Clifton NJ 2013, 1001, 179–188. [Google Scholar] [CrossRef]
- Hu, J.; Ai, B.; Zhu, S.; Wang, Z.; Xia, H.; Jia, W. Electrospun PLGA and PLGA/Gelatin Scaffolds for Tubularized Urethral Replacement: Studies in Vitro and in Vivo. J. Biomater. Appl. 2022, 36, 956–964. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Chen, G.; Komuro, H.; Ushida, T.; Kaneko, S.; Tateishi, T.; Kaneko, M. Tissue-Engineered Urinary Bladder Wall Using PLGA Mesh-Collagen Hybrid Scaffolds: A Comparison Study of Collagen Sponge and Gel as a Scaffold. J. Pediatr. Surg. 2003, 38, 1781–1784. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-Engineered Autologous Bladders for Patients Needing Cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Sack, B.S.; Mauney, J.R.; Estrada, C.R. Silk Fibroin Scaffolds for Urologic Tissue Engineering. Curr. Urol. Rep. 2016, 17, 16. [Google Scholar] [CrossRef]
- Szymkowiak, S.; Sandler, N.; Kaplan, D.L. Aligned Silk Sponge Fabrication and Perfusion Culture for Scalable Proximal Tubule Tissue Engineering. ACS Appl. Mater. Interfaces 2021, 13, 10768–10777. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a Porcine Bladder Acellular Matrix with Well-Preserved Extracellular Bioactive Factors for Tissue Engineering. Tissue Eng.—Part C Methods 2010, 16, 1201–1211. [Google Scholar] [CrossRef]
- Sloff, M.; Simaioforidis, V.; Tiemessen, D.M.; Janke, H.P.; Kortmann, B.B.M.; Roelofs, L.A.J.; Geutjes, P.J.; Oosterwijk, E.; Feitz, W.F.J. Tubular Constructs as Artificial Urinary Conduits. J. Urol. 2016, 196, 1279–1286. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Feng, F.; Men, C.; Yang, D.; Gao, Z.; Zhu, Z.; Cui, Y.; Zhao, H. A Preclinical Study of Cell-Seeded Tubularized Scaffolds Specially Secreting LL37 for Reconstruction of Long Urethral Defects. Anticancer Res. 2017, 37, 4295–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Yang, F.; Chu, G.; Li, X.; Fu, Q.; Zou, M.; Zhao, P.; Lu, G. Characterizing the Inherent Activity of Urinary Bladder Matrix for Adhesion, Migration, and Activation of Fibroblasts as Compared with Collagen-Based Synthetic Scaffold. J. Biomater. Appl. 2022, 29, 8853282221130883. [Google Scholar] [CrossRef]
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Busch, S.; Daum, L.; Stenzl, A.; Amend, B.; et al. Collagen Cell Carriers Seeded with Human Urothelial Cells for Urethral Reconstructive Surgery: First Results in a Xenograft Minipig Model. World J. Urol. 2017, 35, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Sun, X.-L.; Wang, S.-Y.; Yang, X.; Wang, J.-L. Porcine Small Intestinal Submucosa Mesh for Treatment of Pelvic Organ Prolapsed. Chin. Med. J. 2016, 129, 2603–2609. [Google Scholar] [CrossRef]
- Xia, D.; Yang, Q.; Fung, K.-M.; Towner, R.A.; Smith, N.; Saunders, D.; Greenwood-Van Meerveld, B.; Kropp, B.P.; Madihally, S.V.; Lin, H.-K. Immunomodulatory Response of Layered Small Intestinal Submucosa in a Rat Bladder Regeneration Model. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1960–1969. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral Reconstruction with Autologous Urine-Derived Stem Cells Seeded in Three-Dimensional Porous Small Intestinal Submucosa in a Rabbit Model. Stem Cell Res. Ther. 2017, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Xia, D.; Towner, R.A.; Smith, N.; Saunders, D.; Fung, K.-M.; Aston, C.E.; Greenwood-Van Meerveld, B.; Hurst, R.E.; Madihally, S.V.; et al. Reduced Urothelial Regeneration in Rat Bladders Augmented with Permeable Porcine Small Intestinal Submucosa Assessed by Magnetic Resonance Imaging. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1778–1787. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Jiang, Y.-L.; Hu, J.-G.; Zhao, L.-M.; Chen, Q.-Z.; Liang, Y.; Zhang, Y.; Lei, X.-X.; Wang, R.; Lei, Y.; et al. Procyanidins-Crosslinked Small Intestine Submucosa: A Bladder Patch Promotes Smooth Muscle Regeneration and Bladder Function Restoration in a Rabbit Model. Bioact. Mater. 2021, 6, 1827–1838. [Google Scholar] [CrossRef]
- Bury, M.I.; Fuller, N.J.; Sturm, R.M.; Rabizadeh, R.R.; Nolan, B.G.; Barac, M.; Edassery, S.S.; Chan, Y.Y.; Sharma, A.K. The Effects of Bone Marrow Stem and Progenitor Cell Seeding on Urinary Bladder Tissue Regeneration. Sci. Rep. 2021, 11, 2322. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, D.; Yao, H.; Fu, S.; Wei, Z.; Wang, Z.; Xie, M. An Extracellular Matrix-Mimicking, Bilayered, Heterogeneous, Porous, Nanofibrous Scaffold for Anterior Urethroplasty in a Rabbit Model. Biomed. Mater. Bristol Engl. 2020, 15, 065008. [Google Scholar] [CrossRef]
- Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A.; et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. Int. J. Mol. Sci. 2022, 23, 2826. [Google Scholar] [CrossRef] [PubMed]
- Pokrywczynska, M.; Jundzill, A.; Tworkiewicz, J.; Buhl, M.; Balcerczyk, D.; Adamowicz, J.; Kloskowski, T.; Rasmus, M.; Mecinska-Jundzill, K.; Kasinski, D.; et al. Urinary Bladder Augmentation with Acellular Biologic Scaffold-A Preclinical Study in a Large Animal Model. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cao, N.; Fan, S.; Zhang, H.; Shao, H.; Song, L.; Cao, C.; Huang, J.; Zhang, Y. Angiogenesis Potential of Bladder Acellular Matrix Hydrogel by Compounding Endothelial Cells. ACS Appl. Bio Mater. 2019, 2, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, M.; Rahavian, A.; Fallah Karkan, M.; Allameh, F.; Ghiasy, S.; Javanmard, B. Use of Human Amniotic Membrane Repair of Anterior Urethral Defect: First Clinical Report. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2020, 27, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Hou, X.; Zhao, Y.; Chen, B.; Dai, J.; Sun, N. Urethral Tissue Reconstruction Using the Acellular Dermal Matrix Patch Modified with Collagen-Binding VEGF in Beagle Urethral Injury Models. BioMed Res. Int. 2021, 2021, 5502740. [Google Scholar] [CrossRef]
- Atala, A.; Danilevskiy, M.; Lyundup, A.; Glybochko, P.; Butnaru, D.; Vinarov, A.; Yoo, J.J. The Potential Role of Tissue-Engineered Urethral Substitution: Clinical and Preclinical Studies. J. Tissue Eng. Regen. Med. 2017, 11, 3–19. [Google Scholar] [CrossRef]
- Todesco, M.; Zardin, C.; Iop, L.; Palmosi, T.; Capaldo, P.; Romanato, F.; Gerosa, G.; Bagno, A. Hybrid Membranes for the Production of Blood Contacting Surfaces: Physicochemical, Structural and Biomechanical Characterization. Biomater. Res. 2021, 25, 26. [Google Scholar] [CrossRef]
- Reed, A.M.; Potter, J.; Szycher, M. A Solution Grade Biostable Polyurethane Elastomer: ChronoFlex® AR. J. Biomater. Appl. 1994, 8, 210–236. [Google Scholar] [CrossRef]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small Intestinal Submucosa as a Large Diameter Vascular Graft in the Dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Lantz, G.C.; Badylak, S.F.; Coffey, A.C.; Geddes, L.A.; Blevins, W.E. Small Intestinal Submucosa as a Small-Diameter Arterial Graft in the Dog. J. Investig. Surg. 1990, 3, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Borile, G.; Sandrin, D.; Filippi, A.; Anderson, K.I.; Romanato, F. Label-Free Multiphoton Microscopy: Much More Than Fancy Images. Int. J. Mol. Sci. 2021, 22, 2657. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Gintoli, M.; Filippi, A.; Sasso, E.D.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; et al. Multimodal Label-Free Ex Vivo Imaging Using a Dual-Wavelength Microscope with Axial Chromatic Aberration Compensation. J. Biomed. Opt. 2018, 23, 091403. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; Van De Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental Investigation of Collagen Waviness and Orientation in the Arterial Adventitia Using Confocal Laser Scanning Microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, K. LoadSpectra. Available online: https://www.mathworks.com/matlabcentral/fileexchange/57904-loadspectra (accessed on 11 February 2022).
- Council of Europe. 2.6.1. Sterility; European Pharmacopoeia; Council of Europe: London, UK, 2005; pp. 145–149. [Google Scholar]
- C.S.A. ISO 10993-5 in Vitro Cytotoxicity. Int. Organ. 2009, 2007, 1–11. [Google Scholar]
- Lee, R.K.; Abol-Enein, H.; Artibani, W.; Bochner, B.; Dalbagni, G.; Daneshmand, S.; Fradet, Y.; Hautmann, R.E.; Lee, C.T.; Lerner, S.P.; et al. Urinary Diversion after Radical Cystectomy for Bladder Cancer: Options, Patient Selection, and Outcomes: Urinary Diversion after Radical Cystectomy for Bladder Cancer. BJU Int. 2014, 113, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Presicce, F.; Leonardo, C.; Tuderti, G.; Brassetti, A.; Mastroianni, R.; Bove, A.; Misuraca, L.; Anceschi, U.; Ferriero, M.; Gallucci, M.; et al. Late Complications of Robot-Assisted Radical Cystectomy with Totally Intracorporeal Urinary Diversion. World J. Urol. 2021, 39, 1903–1909. [Google Scholar] [CrossRef]
- Johnson, S.C.; Smith, Z.L.; Sack, B.S.; Steinberg, G.D. Tissue Engineering and Conduit Substitution. Urol. Clin. North Am. 2018, 45, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and Engineered Options for Urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Morlacco, A.; Dal Moro, F. Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes 2021, 9, 1643. [Google Scholar] [CrossRef]
- Eberli, D.; Filho, L.F.; Atala, A.; Yoo, J.J. Composite Scaffolds for the Engineering of Hollow Organs and Tissues. Methods 2009, 47, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Ananta, M.; Aulin, C.E.; Sc, M.; Ph, D.; Aibibu, D. A Poly (Lactic Acid-Co-Caprolactone)–Collagen Hybrid for Tissue Engineering Applications. Tissue Eng. Part A 2009, 15, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, E.M.; Micol, L.A.; Houis, S.; Wurm, F.M.; Hilborn, J.; Hubbell, J.A.; Frey, P. A Collagen-Poly(Lactic Acid-Co-ε-Caprolactone) Hybrid Scaffold for Bladder Tissue Regeneration. Biomaterials 2011, 32, 3969–3976. [Google Scholar] [CrossRef]
- Franck, D.; Gil, E.S.; Adam, R.M.; Kaplan, D.L.; Chung, Y.G.; Estrada, C.R.; Mauney, J.R. Evaluation of Silk Biomaterials in Combination with Extracellular Matrix Coatings for Bladder Tissue Engineering with Primary and Pluripotent Cells. PLoS ONE 2013, 8, e56237. [Google Scholar] [CrossRef]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A Bilayered Hybrid Microfibrous PLGA-Acellular Matrix Scaffold for Hollow Organ Tissue Engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Horst, M.; Milleret, V.; Nötzli, S.; Madduri, S.; Sulser, T.; Gobet, R.; Eberli, D. Increased Porosity of Electrospun Hybrid Scaffolds Improved Bladder Tissue Regeneration. J. Biomed. Mater. Res.—Part A 2014, 102, 2116–2124. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Zeiai, S.; Fossum, M.; Hilborn, J.G. Constructs of Electrospun PLGA, Compressed Collagen and Minced Urothelium for Minimally Manipulated Autologous Bladder Tissue Expansion. Biomaterials 2014, 35, 5741–5748. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pokrywczyńska, M.; Tworkiewicz, J.; Kowalczyk, T.; Van Breda, S.V.; Tyloch, D.; Kloskowski, T.; Bodnar, M.; Skopinska-Wisniewska, J.; Marszałek, A.; et al. New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology. PLoS ONE 2016, 11, e0146012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horst, M.; Milleret, V.; Noetzli, S.; Gobet, R.; Sulser, T.; Eberli, D. Polyesterurethane and Acellular Matrix Based Hybrid Biomaterial for Bladder Engineering. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2017, 105, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a Conductive Biocomposite Combining Graphene and Amniotic Membrane for Replacement of the Neuronal Network of Tissue-Engineered Urinary Bladder. Sci. Rep. 2020, 10, 5824. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Kloskowski, T.; Stopel, M.; Gniadek, M.; Rasmus, M.; Balcerczyk, D.; Buhl, M.; Gagat, M.; Antosik, P.; Grzanka, D.; et al. The development of marine biomaterial derived from decellularized squid mantle for potential application as tissue engineered urinary conduit. Mater. Sci. Eng. C 2021, 119, 111579. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Inclusion of Substances of Very High Concerns in the Candidate List; European Chemicals Agency: Helsinki, Finland, 2012; pp. 1–9. [Google Scholar]
- Jundziłł, A.; Kwieciński, P.; Balcerczyk, D.; Kloskowski, T.; Grzanka, D.; Antosik, P.; Meger, K.; Pokrywczyńska, M.; Drewa, T. A tissue-engineered urinary conduit in a porcine urinary diversion model. Sci. Rep. 2021, 11, 16754. [Google Scholar] [CrossRef]
- Drewa, T.; Adamowicz, J.; Sharma, A. Tissue engineering for the oncologic urinary bladder. Nat. Rev. Urol. 2012, 9, 561–572. [Google Scholar] [CrossRef]
- Adamowicz, J.; Van Breda, S.V.; Kloskowski, T.; Juszczak, K.; Pokrywczynska, M.; Drewa, T. Constructing artificial urinary conduits: Current capabilities and future potential. Expert Rev. Med. Devices 2019, 16, 135–144. [Google Scholar] [CrossRef]
- Tian, H.; Bharadwaj, S.; Liu, Y.; Ma, P.X.; Atala, A.; Zhang, Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: Potential for urological tissue engineering. Tissue Eng.—Part A 2010, 16, 1769–1779. [Google Scholar] [CrossRef] [Green Version]
- Joachimiak, R.; Bajek, A.; Cieslak, Z.; Drewa, T.A.; Gagat, M.; Grzanka, A.; Marszałek, A.; Debski, R. C53 Hair Follicle Stem Cells and Bone Marrow Mesenchymal Stem Cells in Bladder Regeneration. Eur. Urol. Suppl. 2010, 9, 632–633. [Google Scholar] [CrossRef]
- Ning, J.; Li, C.; Li, H.; Chang, J. Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology 2011, 63, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloskowski, T.; Kowalczyk, T.; Nowacki, M.; Drewa, T. Tissue engineering and ureter regeneration: Is it possible? Int. J. Artif. Organs 2013, 36, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Drewa, T. The Artificial Conduit for Urinary Diversion in Rats: A Preliminary Study. Transplant. Proc. 2007, 39, 1647–1651. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarin, M.; Todesco, M.; Sandrin, D.; Romanato, F.; Bagno, A.; Morlacco, A.; Dal Moro, F. A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers. J. Funct. Biomater. 2022, 13, 222. https://doi.org/10.3390/jfb13040222
Casarin M, Todesco M, Sandrin D, Romanato F, Bagno A, Morlacco A, Dal Moro F. A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers. Journal of Functional Biomaterials. 2022; 13(4):222. https://doi.org/10.3390/jfb13040222
Chicago/Turabian StyleCasarin, Martina, Martina Todesco, Deborah Sandrin, Filippo Romanato, Andrea Bagno, Alessandro Morlacco, and Fabrizio Dal Moro. 2022. "A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers" Journal of Functional Biomaterials 13, no. 4: 222. https://doi.org/10.3390/jfb13040222