Effect of Surface Pre-Reacted Glass Ionomer Containing Dental Sealant on the Inhibition of Enamel Demineralization
Abstract
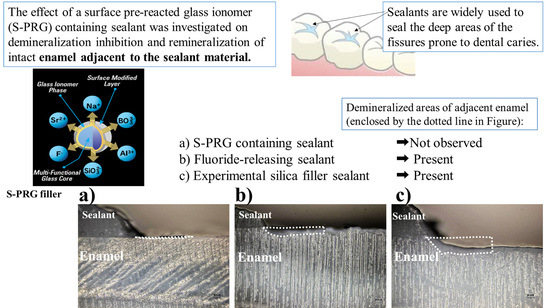
:1. Introduction
2. Materials and Methods
2.1. Sealant Materials
2.2. Specimen Preparation and pH Cycling
2.3. Inhibition of Demineralization
2.4. pH and Buffering Effect
2.5. Statistical Analysis
3. Demineralization after pH Cycling
4. FE-SEM/EDS Findings after Demineralization
5. pH Measurement
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feathestone, J.D.B. Remineralization, the natural caries repair process—The need for new approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef]
- O’keefe, E. Early childhood caries. Evid. Based Dent. 2013, 14, 40–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, B.A.; Eklund, S.A. Dentistry, Dental Practice, and the Community, 6th ed.; Elsevier Saunders: St. Louis, MO, USA, 2005. [Google Scholar]
- Trairatvorakul, C.; Kladkaew, S.; Songsiripradabboon, S. Active management of incipient caries and choice of materials. J. Dent. Res. 2008, 87, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Nikaido, T.; Alireza, S.; Takagaki, T.; Chen, J.H.; Tagami, J. Phosphoric acid-etching promotes bond strength and formation of acid-base resistant zone on enamel. Oper Dent. 2013, 38, 82–90. [Google Scholar] [CrossRef]
- Ma, S.; Imazato, S.; Chen, J.; Mayanagi, G.; Takahashi, N.; Ishimoto, T. Effect of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent. Mater. J. 2012, 31, 909–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiiya, T.; Mukai, Y.; Tomiyama, K.; Teranaka, T. Anti-demineralization effect of a novel fluoride-releasing varnish on dentin. Am. J. Dent. 2012, 25, 347–350. [Google Scholar]
- Shimazu, K.; Ogata, K.; Karibe, H. Caries-preventive effect of fissure sealant containing surface reaction-type glass ionomer filler and bonded by self-etching primer. J. Clin. Pediatr. Dent. 2012, 36, 343–347. [Google Scholar] [CrossRef]
- Kaga, M.; Kakuda, S.; Ida, Y.; Toshima, H.; Hashimoto, M.; Endo, K.; Sano, H. Inhibition of enamel demineralization by buff- ering effect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 2014, 122, 78–83. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kambara, M. Effects of ion-releasing tooth-coating material on demineralization of bovine tooth enamel. Int. J. Dent. 2014, 2014, 463149. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamada, A.; Saito, K.; Hino, R.; Sugawara, Y.; Ono, M.; Naruse, M.; Arakaki, M.; Fukumoto, S. Application of a tooth-surface coating material containing pre-reacted glass- ionomer fillers for caries prevention. Pediatr. Dent. J. 2015, 25, 72–78. [Google Scholar] [CrossRef]
- Ito, S.; Iijima, M.; Hashimoto, M.; Tsukamoto, N.; Mizoguchi, I.; Saito, T. Effects of surface pre-reacted glass-ionomer fillers on mineral induction by phosphoprotein. J. Dent. 2011, 39, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Cv, E.; Li, M.; Niwano, K.; Ab, N.; Okamoto, A.; Honda, N.; Iwaku, M. Effect of fluoride mouth rinse on fluoride releasing and recharging from aesthetic dental materials. Dent. Mater. J. 2002, 21, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Itota, T.; Carrick, T.E.; Yoshiyama, M.; McCabe, J.F. Fluoride release and recharge in giomer, compomer and resin composite. Dent. Mater. 2004, 20, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Sayed, M.; Hill, R.; Tagami, J.; Hayashi, F. Interactions of boron released from surface pre-reacted glass ionomer with enamel/dentin and its effect on pH. Sci. Rep. 2021, 3, 15734. [Google Scholar] [CrossRef]
- Zhou, Y.; Hiraishi, N.; Shimada, Y.; Wang, G.; Tagami, J.; Feng, X. Evaluation of tooth demineralization and interfacial bacterial penetration around resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2021, 37, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sayed, M.; Takahashi, M.; Matin, K.; Hiraishi, N.; Nikaido, T.; Burrow, M.F.; Tagami, J. Effects of a surface prereacted glass-ionomer filler coating material on biofilm formation and inhibition of dentin demineralization. Clin. Oral Investig. 2021, 25, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Dedhiya, M.G.; Young, F.; Higuchi, W.I. Mechanism for the retardation of the acid dissolution rate of hydroxyapatite by strontium. J. Dent. Res. 1973, 52, 1097–1109. [Google Scholar] [CrossRef]
- Iijima, Y.; Koulourides, T. Fluoride incorporation into and retention in remineralized enamel. J. Dent. Res. 1989, 68, 1289–1292. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Saranathan, S.; Cai, F.; Cross, K.J.; Reynolds, E.C. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008, 42, 88–97. [Google Scholar] [CrossRef] [PubMed]
- de Souza Penha, K.J.; de Oliveira Roma, F.R.V.; Filho, E.M.M.; Ribeiro, C.C.C.; Leily Macedo Firoozmand. Bioactive self-etching sealant on newly erupted molar s: A split-mouth clinical trial. J. Dent. 2021, 115, 103857. [Google Scholar] [CrossRef] [PubMed]
- Ntaoutidou, S.; Arhakis, A.; Tolidis, K.; Kotsanos, N. Clinical evaluation of a surface pre-reacted glass (S-PRG) filler-containing dental sealant placed with a self-etching primer/adhesive. Eur. Arch. Paediatr. Dent. 2018, 19, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.S.; Hara, A.T.; Paes Leme, A.F.; Cury, J.A. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz. Dent. J. 2008, 19, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kajiwara, D.; Minamikawa, H.; Kakuda, S.; Hashimoto, M.; Wakayama, Y. Ion release and buffering capacity of S-PRG filler-containing pit and fissure sealant in lactic acid. Nano Biomed. 2011, 3, 275–278. [Google Scholar]
- Swift, E.J.; Perdigão, J.; Heymann, H.O. Bonding to enamel and dentin. a brief history and state of the art, 1995. Quintessence Int. 1995, 26, 95–110. [Google Scholar]
- Dionysopoulos, D.; Sfeikos, T.; Tolidis, K. Fluoride release and recharging ability of new dental sealants. Eur. Arch. Paediatr. Dent. 2016, 17, 45–51. [Google Scholar] [CrossRef]
- Garcia-Godoy, F.; Abarzua, I.; De Goes, M.F.; Chan, D.C. Fluoride release from fissure sealants. J. Clin. Pediatr. Dent. 1997, 22, 45–49. [Google Scholar]
- Bertacchini, S.M.; Abate, P.F.; Blank, A.; Baglieto, M.F.; Macchi, R.L. Solubility and fluoride release in ionomers and compomers. Quintessence Int. 1999, 30, 193–197. [Google Scholar]
- Rajtboriraks, D.; Nakornchai, S.; Bunditsing, P.; Surarit, R.; Iemjarern, P. Plaque and saliva fluoride levels after placement of fluoride releasing pit and fissure sealants. Pediatr. Dent. 2004, 26, 63–66. [Google Scholar]
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Helvatzoglou-Antoniades, M.; Kotsanos, N. Fluoride release and recharge ability of contemporary fluoride-containing restorative materials and dental adhesives. Dent. Mater. J. 2013, 32, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, K.; Ogata, K.; Karibe, H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler. Dent. Mater. J. 2011, 30, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Mishima, H.; Kozawa, Y. SEM and EDS analysis of calcospherites in human teeth. Eur. J. Oral Sci. 1998, 106, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.C.; Mount, G.; Mc Intyre, J.; Tuisuva, J.; Von Doussa, R.J. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: An in vivo study. J. Dent. 2006, 34, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Nakagaki, H.; Kato, K.; Hung, P.A.; Inukai, J.; Tsuboi, S.; Hirose, M.N.; Igarashi, S.; Robinson, C. Effect of strontium in combination with fluoride on enamel remineralization in vitro. Arch. Oral Biol. 2008, 53, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okiji, T. Evaluationoftheionsrelease/incorporationof the prototype S-PRG filler-containing endodontic sealer. Dent. Mater. J. 2011, 30, 898–903. [Google Scholar] [CrossRef] [Green Version]
- Cattani-Lorente, M.A.; Dupuis, V.; Moya, F.; Payan, J.; Meyer, J.M. Comparative study of the physical properties of a polyacid- modified composite resin and a resin-modified glass ionomer cement. Dent. Mater. 1999, 15, 21–32. [Google Scholar] [CrossRef]
- Czarnecka, B.; Nicholson, J.W. Ion release by resin-modified glass-ionomer cements into water and lactic acid solutions. J. Dent. 2006, 34, 539–543. [Google Scholar] [CrossRef]
- Geneste, G.; Bouyer, F.; Gin, S. Hydrogen–sodium interdiffusion in borosilicate glasses investigated from first principles. J. Non-Cryst. Solids 2006, 352, 3147–3152. [Google Scholar] [CrossRef]
- Sayed, M.; Hiraishi, N.; Matin, K.; Abdou, A.; Burrow, M.; Tagami, J. Effect of silver-containing agents on the ultra-structural morphology of dentinal collagen. Dent Mater. 2020, 36, 936–944. [Google Scholar] [CrossRef]
- Robinson, C.; Connell, S.; Kirkham, J.; Brookes, S.J.; Shore, R.C.; Smith, A.M. The effect of fluoride on the developing tooth. Caries Res. 2004, 38, 268–276. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials-Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Dionysopoulos, D. The effect of fluoride-releasing restorative materials on inhibition of secondary caries formation. Fluoride 2014, 47, 258–265. [Google Scholar]







| Brand | Abbreviations | Batch Number | Chemical Composition | Manufacturer |
|---|---|---|---|---|
| BeautiSealant | BTS | 042157 | UDMA, TEGDMA S-PRG filler | Shofu, Kyoto, Japan |
| BeautiSealant Primer | 071954 | acetone, distilled water, carboxylic acid monomer, phosphonic acid monomers and others | Shofu, Kyoto, Japan | |
| TeethmateF-1 2.0 | TMF | 2H0048 | MDP, MF-MMA, TEGDMA, HEMA | Kuraray Noritake Dental, Tokyo, Japan |
| K-etchant GEL | 1N0122 | Phosphoric acid, water, colloidal silica, dye | Kuraray Noritake Dental. Tokyo, Japan | |
| Silica-filler Sealant | SS | 210323-s3 | UDMA, TEGDMA, silica filler | Shofu, Kyoto, Japan |
| Sealant Material | [F] Mass (%) (Mean ± SD) |
|---|---|
| BTS | 4.7 ± 0.2 a |
| TMF | 2.0 ± 0.2 b |
| SS | 0.0 ± 0.0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, Y.; Sayed, M.; Hiraishi, N.; Al-Haj Husain, N.; Tagami, J.; Özcan, M.; Shimada, Y. Effect of Surface Pre-Reacted Glass Ionomer Containing Dental Sealant on the Inhibition of Enamel Demineralization. J. Funct. Biomater. 2022, 13, 189. https://doi.org/10.3390/jfb13040189
Ogawa Y, Sayed M, Hiraishi N, Al-Haj Husain N, Tagami J, Özcan M, Shimada Y. Effect of Surface Pre-Reacted Glass Ionomer Containing Dental Sealant on the Inhibition of Enamel Demineralization. Journal of Functional Biomaterials. 2022; 13(4):189. https://doi.org/10.3390/jfb13040189
Chicago/Turabian StyleOgawa, Yuko, Mahmoud Sayed, Noriko Hiraishi, Nadin Al-Haj Husain, Junji Tagami, Mutlu Özcan, and Yasushi Shimada. 2022. "Effect of Surface Pre-Reacted Glass Ionomer Containing Dental Sealant on the Inhibition of Enamel Demineralization" Journal of Functional Biomaterials 13, no. 4: 189. https://doi.org/10.3390/jfb13040189