Determination of PAHs, PAH-Derivatives and Other Concerning Substances in Posidonia oceanica Seagrass and Marine Sediments by High Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
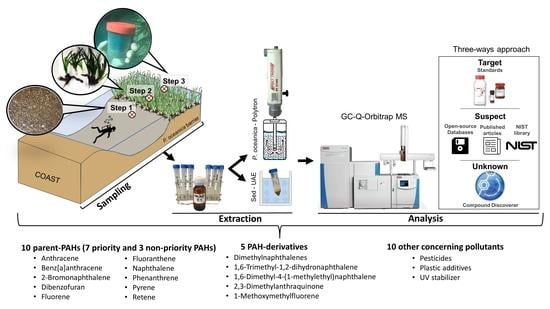
2.2. Study Area and Sampling
2.3. Sample Pretreatment
2.4. Extraction Procedure
2.5. GC-Q-Orbitrap MS Parameters
2.6. Method Validation
2.7. Analysis of the Environmental Samples: Target and Non-Target Approaches
2.7.1. Target Analysis
2.7.2. Suspect Analysis
2.7.3. Unknown Analysis
3. Results and Discussion
3.1. Extraction Procedure Optimization and Validation
3.2. Application to Environmental Samples
3.2.1. Target Analysis
Biotic Compartment: Leaves and Rhizomes
Abiotic Compartment: Sediment
Compartmentation of the Target PAHs
PAH Source Identification
3.2.2. Suspect Analysis
3.2.3. Unknown Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Calvo, S.; Clabaut, P.; Harmelin-vivien, M.; Mateo, M.A.; Montefalcone, M.; et al. Climate change and Mediterranean seagrass meadows: A synopsis for environmental managers. Mediterr. Mar. Sci. 2014, 15, 462. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- IUCN. Manual for the Creation of Blue Carbon Projects in Europe and the Mediterranean; Otero, M., Ed.; IUCN: Málaga, Spain, 2021; 144p. [Google Scholar]
- Paoli, C.; Povero, P.; Burgos, E.; Dapueto, G.; Fanciulli, G.; Massa, F.; Scarpellini, P.; Vassallo, P. Natural capital and environmental flows assessment in marine protected areas: The case study of Liguria region (NW Mediterranean Sea). Ecol. Modell. 2018, 368, 121–135. [Google Scholar] [CrossRef]
- Rigo, I.; Paoli, C.; Dapueto, G.; Pergent-Martini, C.; Pergent, G.; Oprandi, A.; Montefalcone, M.; Bianchi, C.N.; Morri, C.; Vassallo, P. The Natural Capital Value of the Seagrass Posidonia oceanica in the North-Western Mediterranean. Diversity 2021, 13, 499. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed]
- Marbà, N.; Díaz-Almela, E.; Duarte, C.M. Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Monnier, B.; Lehmann, L.; Barralon, E.; Pergent, G. Major regression of Posidonia oceanica meadows in relation with recreational boat anchoring: A case study from Sant’Amanza bay. J. Sea Res. 2022, 188, 102258. [Google Scholar] [CrossRef]
- Bucalossi, D.; Leonzio, C.; Casini, S.; Fossi, M.C.; Marsili, L.; Ancora, S.; Wang, W.; Scali, M. Application of a suite of biomarkers in Posidonia oceanica (L.) Delile to assess the ecotoxicological impact on the coastal environment. Mar. Environ. Res. 2006, 62, S327–S331. [Google Scholar] [CrossRef] [PubMed]
- Moschino, V.; Del Negro, P.; De Vittor, C.; Da Ros, L. Biomonitoring of a polluted coastal area (Bay of Muggia, Northern Adriatic Sea): A five-year study using transplanted mussels. Ecotoxicol. Environ. Saf. 2016, 128, 1–10. [Google Scholar] [CrossRef]
- Trisciani, A.; Corsi, I.; Torre, C.D.; Perra, G.; Focardi, S. Hepatic biotransformation genes and enzymes and PAH metabolites in bile of common sole (Solea solea, Linnaeus, 1758) from an oil-contaminated site in the Mediterranean Sea: A field study. Mar. Pollut. Bull. 2011, 62, 806–814. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Q.; Zhu, B.; Lan, Y.; Duan, S. Long-term exposure to benzo[a]pyrene affects sexual differentiation and embryos toxicity in three generations of marine medaka (Oryzias melastigma). Int. J. Environ. Res. Public Health 2020, 17, 970. [Google Scholar] [CrossRef] [PubMed]
- Kurelec, B. The Genotoxic Disease Syndrome. Mar. Environ. Res. 1993, 35, 341–348. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Drwal, E.; Rak, A.; Gregoraszczuk, E.L. Review: Polycyclic aromatic hydrocarbons (PAHs)—Action on placental function and health risks in future life of newborns. Toxicology 2019, 411, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive No. 2013/39/EC. Off. J. Eur. Union 2013, L226, 1–17.
- ICES, International Council for the Exploration of the Sea. Report of the ICES Advisory Committee on the Marine Environment. In ICES Advice; ICES: Copenhagen, Denmark, 2004; Volume 1. [Google Scholar]
- OSPAR Commission. OSPAR/ICES Workshop on the evaluation and update of background reference concentrations (B/RCs) and ecotoxicological assessement criteria (EACs) and how these assessement tools should be used in assessing contaminants in water, sediment and biota. In Workshop; OSPAR Commission: The Hague, The Netherlands, 2004; Volume 913, pp. 1–169. [Google Scholar]
- Gonul, L.T.; Kucuksezgin, F. Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments from the Eastern Aegean: Assessment and source recognition of petroleum hydrocarbons. Environ. Sci. Pollut. Res. 2012, 19, 31–41. [Google Scholar] [CrossRef]
- Froger, C.; Ayrault, S.; Gasperi, J.; Caupos, E.; Monvoisin, G.; Evrard, O.; Quantin, C. Innovative combination of tracing methods to differentiate between legacy and contemporary PAH sources in the atmosphere-soil-river continuum in an urban catchment (Orge River, France). Sci. Total Environ. 2019, 669, 448–458. [Google Scholar] [CrossRef]
- Albaigés, J. Persistent Organic Pollutants in the Mediterranean Sea. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 5, pp. 89–149. [Google Scholar]
- Merhaby, D.; Rabodonirina, S.; Net, S.; Ouddane, B.; Halwani, J. Overview of sediments pollution by PAHs and PCBs in mediterranean basin: Transport, fate, occurrence, and distribution. Mar. Pollut. Bull. 2019, 149, 110646. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Sunyer-Caldú, A.; Díaz-Cruz, M.S.; Frank-Comas, A.; García-Márquez, M.G.; Tovar-Sánchez, A. Mediterranean seagrass Posidonia oceanica accumulates sunscreen UV filters. Mar. Pollut. Bull. 2022, 176, 113417. [Google Scholar] [CrossRef] [PubMed]
- De los Santos, C.B.; Krång, A.-S.; Infantes, E. Microplastic retention by marine vegetated canopies: Simulations with seagrass meadows in a hydraulic flume. Environ. Pollut. 2021, 269, 116050. [Google Scholar] [CrossRef]
- Jebara, A.; Lo Turco, V.; Potortì, A.G.; Bartolomeo, G.; Ben Mansour, H.; Di Bella, G. Organic pollutants in marine samples from Tunisian coast: Occurrence and associated human health risks. Environ. Pollut. 2021, 271, 116266. [Google Scholar] [CrossRef]
- Mauro, L.; Paola, G.; Margherita, V.; Rugiada, R.; Francesca, B.; Primo, M.; Duccio, S.; Enrica, F. Human impact on a small barrier reef meadow of Posidonia oceanica (L.) Delile on the north Tyrrhenian coast (Italy). Mar. Pollut. Bull. 2013, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pergent, G.; Labbe, C.; Lafabrie, C.; Kantin, R.; Pergent-Martini, C. Organic and inorganic human-induced contamination of Posidonia oceanica meadows. Ecol. Eng. 2011, 37, 999–1002. [Google Scholar] [CrossRef]
- Apostolopoulou, M.V.; Monteyne, E.; Krikonis, K.; Pavlopoulos, K.; Roose, P.; Dehairs, F. Monitoring polycyclic aromatic hydrocarbons in the Northeast Aegean Sea using Posidonia oceanica seagrass and synthetic passive samplers. Mar. Pollut. Bull. 2014, 87, 338–344. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Kang, H.-J.; Jung, Y.; Kwon, J.-H. Changes in ecotoxicity of naphthalene and alkylated naphthalenes during photodegradation in water. Chemosphere 2019, 222, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581, 237–257. [Google Scholar] [CrossRef]
- Chibwe, L.; Geier, M.C.; Nakamura, J.; Tanguay, R.L.; Aitken, M.D.; Simonich, S.L.M. Aerobic Bioremediation of PAH Contaminated Soil Results in Increased Genotoxicity and Developmental Toxicity. Environ. Sci. Technol. 2015, 49, 13889–13898. [Google Scholar] [CrossRef] [PubMed]
- Astudillo-Pascual, M.; Domínguez, I.; Aguilera, P.A.; Garrido Frenich, A. New phenolic compounds in Posidonia oceanica seagrass: A comprehensive array using high resolution mass spectrometry. Plants 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Schrader, S.; Moeder, M. Determination of organic priority pollutants and emerging compounds in wastewater and snow samples using multiresidue protocols on the basis of microextraction by packed sorbents coupled to large volume injection gas chromatography-mass spectrometry analy. J. Chromatogr. A 2010, 1217, 6002–6011. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2009/90/EC. Directive 2009/90/EC of 31 July 2009 laying down, pursuant to Directive 2000/60/EC of the European Parliament and of the Council, technical specifications for chemical analysis and monitoring of water status. Off. J. Eur. Union 2009, L201, 36–38.
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. EUR 28099; Publication Office of the European Union: Luxemburg, 2016; ISBN 9789279617683. [Google Scholar] [CrossRef]
- Domínguez, I.; Arrebola, F.J.; Martínez Vidal, J.L.; Garrido Frenich, A. Assessment of wastewater pollution by gas chromatography and high resolution Orbitrap mass spectrometry. J. Chromatogr. A 2020, 1619, 460964. [Google Scholar] [CrossRef]
- Penteado, J.C.; Bruns, R.E.; De Carvalho, L.R.F. Factorial design optimization of solid phase microextraction conditons for gas chromatography–mass spectrometry (GC–MS) analysis of linear alkylbenzenes (LABs) in detergents. Anal. Chim. Acta 2006, 562, 152–157. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.Z.; Liang, B.; Shen, R.L.; Zeng, E.Y. Assessment of aquatic wastewater pollution in a highly industrialized zone with sediment linear alkylbenzenes. Environ. Toxicol. Chem. 2012, 31, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Pieke, E.N.; Granby, K.; Trier, X.; Smedsgaard, J. A framework to estimate concentrations of potentially unknown substances by semi-quantification in liquid chromatography electrospray ionization mass spectrometry. Anal. Chim. Acta 2017, 975, 30–41. [Google Scholar] [CrossRef]
- Kwiecien, N.W.; Bailey, D.J.; Rush, M.J.P.; Cole, J.S.; Ulbrich, A.; Hebert, A.S.; Westphall, M.S.; Coon, J.J. High-Resolution Filtering for Improved Small Molecule Identification via GC/MS. Anal. Chem. 2015, 87, 8328–8335. [Google Scholar] [CrossRef]
- Barco-Bonilla, N.; Nieto-García, A.J.; Romero-González, R.; Martínez Vidal, J.L.; Frenich, A.G. Simultaneous and highly sensitive determination of PCBs and PBDEs in environmental water and sediments by gas chromatography coupled to high resolution magnetic sector mass spectrometry. Anal. Methods 2015, 7, 3036–3047. [Google Scholar] [CrossRef]
- Rivoira, L.; Castiglioni, M.; Nurra, N.; Battuello, M.; Sartor, R.M.; Favaro, L.; Bruzzoniti, M.C. Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Seawater, Sediment and Biota of Neritic Ecosystems: Occurrence and Partition Study in Southern Ligurian Sea. Appl. Sci. 2022, 12, 2564. [Google Scholar] [CrossRef]
- León, V.M.; Viñas, L.; Concha-Graña, E.; Fernández-González, V.; Salgueiro-González, N.; Moscoso-Pérez, C.; Muniategui-Lorenzo, S.; Campillo, J.A. Identification of contaminants of emerging concern with potential environmental risk in Spanish continental shelf sediments. Sci. Total Environ. 2020, 742, 140505. [Google Scholar] [CrossRef]
- Balcioğlu, E.B.; Gönülal, O.; Güreşen, S.O.; Aksu, A.; Öztürk, B. Comparison and origins of polycyclic aromatic hydrocarbons (PAHs) in the entrance and the exit of the Turkish Straits System (TSS). Mar. Pollut. Bull. 2018, 136, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.; Salvagio Manta, D.; Al-Tayeb Sharif, E.A.; D’Agostino, F.; Traina, A.; Quinci, E.M.; Giaramita, L.; Monastero, C.; Benothman, M.; Sprovieri, M. Marine pollution in the Libyan coastal area: Environmental and risk assessment. Mar. Pollut. Bull. 2018, 128, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.; Di Martino, V. Trace element compartmentation in the seagrass Posidonia oceanica and biomonitoring applications. Mar. Pollut. Bull. 2017, 116, 196–203. [Google Scholar] [CrossRef]
- Ott, J.A. Growth and Production in Posidonia Oceanica (L.) Delile. Mar. Ecol. 1980, 1, 47–64. [Google Scholar] [CrossRef]
- Pergent, G.; Boudouresque, C.-F.; Crouzet, A.; Meinesz, A. Cyclic Changes along Posidonia oceanica rhizomes (Lepidochronology): Present State and Perspectives. Mar. Ecol. 1989, 10, 221–230. [Google Scholar] [CrossRef]
- Komada, T.; Schofield, O.M.E.; Reimers, C.E. Fluorescence characteristics of organic matter released from coastal sediments during resuspension. Mar. Chem. 2002, 79, 81–97. [Google Scholar] [CrossRef]
- Mandalakis, M.; Polymenakou, P.N.; Tselepides, A.; Lampadariou, N. Distribution of aliphatic hydrocarbons, polycyclic aromatic hydrocarbons and organochlorinated pollutants in deep-sea sediments of the southern Cretan margin, eastern Mediterranean Sea: A baseline assessment. Chemosphere 2014, 106, 28–35. [Google Scholar] [CrossRef]
- Mostert, M.M.R.; Ayoko, G.A.; Kokot, S. Application of chemometrics to analysis of soil pollutants. TrAC Trends Anal. Chem. 2010, 29, 430–445. [Google Scholar] [CrossRef]
- Davis, E.; Walker, T.R.; Adams, M.; Willis, R.; Norris, G.A.; Henry, R.C. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in small craft harbor (SCH) surficial sediments in Nova Scotia, Canada. Sci. Total Environ. 2019, 691, 528–537. [Google Scholar] [CrossRef]
- Rocher, V.; Azimi, S.; Moilleron, R.; Chebbo, G. Hydrocarbons and heavy metals in the different sewer deposits in the “Le Marais” catchment (Paris, France): Stocks, distributions and origins. Sci. Total Environ. 2004, 323, 107–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-C.; Sun, S.; Ma, H.-Q.; Liu, Y. Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Jiaozhou Bay, Qingdao, China. Mar. Pollut. Bull. 2006, 52, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Reina, A.J.; López-Ruiz, R.; Garrido Frenich, A.; Arrebola, F.J.; Romero-González, R. Co-formulants in plant protection products: An analytical approach to their determination by gas chromatography–high resolution mass accuracy spectrometry. Talanta 2021, 234, 122641. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; McConnell, L.L.; Hapeman, C.J.; Nguyen, A.; Schmidt, W.F.; Howard, C.J. Volatile organic compounds in pesticide formulations: Methods to estimate ozone formation potential. Atmos. Environ. 2011, 45, 2404–2412. [Google Scholar] [CrossRef]
- Kruve, A.; Kiefer, K.; Hollender, J. Benchmarking of the quantification approaches for the non-targeted screening of micropollutants and their transformation products in groundwater. Anal. Bioanal. Chem. 2021, 413, 1549–1559. [Google Scholar] [CrossRef]
- Been, F.; Kruve, A.; Vughs, D.; Meekel, N.; Reus, A.; Zwartsen, A.; Wessel, A.; Fischer, A.; ter Laak, T.; Brunner, A.M. Risk-based prioritization of suspects detected in riverine water using complementary chromatographic techniques. Water Res. 2021, 204, 117612. [Google Scholar] [CrossRef]
- Čelić, M.; Jaén-Gil, A.; Briceño-Guevara, S.; Rodríguez-Mozaz, S.; Gros, M.; Petrović, M. Extended suspect screening to identify contaminants of emerging concern in riverine and coastal ecosystems and assessment of environmental risks. J. Hazard. Mater. 2021, 404, 124102. [Google Scholar] [CrossRef]
- Jin, H.; Hao, J.; Yang, J.; Guo, J.; Zhang, Y.; Cao, C.C.; Farooq, A. Experimental and kinetic modeling study of α-methyl-naphthalene pyrolysis: Part II. PAH formation. Combust. Flame 2021, 233, 111530. [Google Scholar] [CrossRef]
- Bateni, F.; Mehdinia, A.; Lundin, L.; Hashtroudi, M.S. Distribution, source and ecological risk assessment of polycyclic aromatic hydrocarbons in the sediments of northern part of the Persian Gulf. Chemosphere 2022, 295, 133859. [Google Scholar] [CrossRef]
- Masood, N.; Zakaria, M.P.; Halimoon, N.; Aris, A.Z.; Magam, S.M.; Kannan, N.; Mustafa, S.; Ali, M.M.; Keshavarzifard, M.; Vaezzadeh, V.; et al. Anthropogenic waste indicators (AWIs), particularly PAHs and LABs, in Malaysian sediments: Application of aquatic environment for identifying anthropogenic pollution. Mar. Pollut. Bull. 2016, 102, 160–175. [Google Scholar] [CrossRef]
- Fox, M.A.; Olive, S. Photooxidation of Anthracene on Atmospheric Particulate Matter. Science 1979, 205, 582–583. [Google Scholar] [CrossRef]
- Kamens, R.M.; Guo, J.; Perry, J.M.; Karam, H.; Stockburger, L. The Behavior of Oxygenated Polycyclic Aromatic Hydrocarbons on Atmospheric Soot Particles. Environ. Sci. Technol. 1989, 23, 801–806. [Google Scholar] [CrossRef]
- Idowu, O.; Carbery, M.; O’Connor, W.; Thavamani, P. Speciation and source apportionment of polycyclic aromatic compounds (PACs) in sediments of the largest salt water lake of Australia. Chemosphere 2020, 246, 125779. [Google Scholar] [CrossRef]
- Idowu, O.; Tran, T.K.A.; Baker, P.; Farrel, H.; Zammit, A.; Semple, K.T.; O’Connor, W.; Thavamani, P. Bioavailability of polycyclic aromatic compounds (PACs) to the Sydney rock oyster (Saccostrea glomerata) from sediment matrices of an economically important Australian estuary. Sci. Total Environ. 2020, 736, 139574. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Saborit, J.M.; Alam, M.S.; Godri Pollitt, K.J.; Stark, C.; Harrison, R.M. Analysis of atmospheric concentrations of quinones and polycyclic aromatic hydrocarbons in vapour and particulate phases. Atmos. Environ. 2013, 77, 974–982. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, X.; Ma, Y.; Ma, J.; Zheng, M.; Shao, M. Concentrations and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in the atmosphere of North China, and the transformation from PAHs to NPAHs. Environ. Pollut. 2015, 196, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Uryu, M.; Hiraga, T.; Koga, Y.; Saito, Y.; Murakami, K.; Itami, K. Synthesis of Polybenzoacenes: Annulative Dimerization of Phenylene Triflate by Twofold C−H Activation. Angew. Chem. 2020, 132, 6613–6616. [Google Scholar] [CrossRef]
- Ellickson, K.M.; McMahon, C.M.; Herbrandson, C.; Krause, M.J.; Schmitt, C.M.; Lippert, C.J.; Pratt, G.C. Analysis of polycyclic aromatic hydrocarbons (PAHs) in air using passive sampling calibrated with active measurements. Environ. Pollut. 2017, 231, 487–496. [Google Scholar] [CrossRef]
- Balmer, J.E.; Hung, H.; Yu, Y.; Letcher, R.J.; Muir, D.C.G. Sources and environmental fate of pyrogenic polycyclic aromatic hydrocarbons (PAHs) in the Arctic. Emerg. Contam. 2019, 5, 128–142. [Google Scholar] [CrossRef]
- Leppänen, H.; Oikari, A. Occurrence of retene and resin acids in sediments and fish bile from a lake receiving pulp and paper mill effluents. Environ. Toxicol. Chem. 1999, 18, 1498–1505. [Google Scholar] [CrossRef]
- Eriksson, A.N.M.; Rigaud, C.; Krasnov, A.; Wincent, E.; Vehniäinen, E.R. Exposure to retene, fluoranthene, and their binary mixture causes distinct transcriptomic and apical outcomes in rainbow trout (Oncorhynchus mykiss) yolk sac alevins. Aquat. Toxicol. 2022, 244, 106083. [Google Scholar] [CrossRef] [PubMed]
- Thuy, H.T.T.; Loan, T.T.C.; Phuong, T.H. The potential accumulation of polycyclic aromatic hydrocarbons in phytoplankton and bivalves in Can Gio coastal wetland, Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 17240–17249. [Google Scholar] [CrossRef] [PubMed]
- Gerstenbacher, C.M.; Finzi, A.C.; Rotjan, R.D.; Novak, A.B. A review of microplastic impacts on seagrasses, epiphytes, and associated sediment communities. Environ. Pollut. 2022, 303, 119108. [Google Scholar] [CrossRef] [PubMed]
- Borovec, O.; Vohník, M. Ontogenetic transition from specialized root hairs to specific root-fungus symbiosis in the dominant Mediterranean seagrass Posidonia oceanica. Sci. Rep. 2018, 8, 10773. [Google Scholar] [CrossRef] [PubMed]
- Garcias-Bonet, N.; Arrieta, J.M.; de Santana, C.N.; Duarte, C.M.; Marbà, N. Endophytic bacterial community of a Mediterranean marine angiosperm (Posidonia oceanica). Front. Microbiol. 2012, 3, 342. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- Hutniczak, B.; Kronbak, L.G. The Two-sector Economic Problem Of Persistent Organic Pollution and Baltic Sea Salmon Fisheries. Consilience 2011, 6, 113–130. [Google Scholar]
- Lawrence, A.J.; Elliot, M. Effects of Pollution on Fish: Molecular Effects and Population Responses; Lawrence, A.J., Hemingway, K.L., Eds.; Blackwell Science: Oxford, UK, 2003; ISBN 0470999683. [Google Scholar]


| Matrix | Analyte | EE2 | EE4 | RM1 | RM2 | RM3 | RM4 | RM5 | ALM1 | ALM2 | ALM3 d | CG2 | CG3 | CG4 | C2 | V2 | ALI1 | ALI2 d | ALI3 | ALI4 | ALI5 | ALI6 | ALI7 | Detection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Naph | 0.7 | 0.9 | 0.7 | 1.2 | 0.9 | 0.9 | 0.8 | 1.0 | 1.2 | 0.7 | 0.9 | 0.8 | 0.9 | 1.0 | 0.9 | * | 1.1 | 0.9 | 1.2 | 1.2 | 1.0 | 1.2 | 100 |
| Flu | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 7.3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 5 | |
| Phe | 7.2 | 9.2 | 6.7 | 9.9 | 18.1 | 17.2 | 12.9 | 11.9 | 12.6 | 17.3 | 6.1 | 21.2 | 13.6 | 15.9 | 4.9 | 11.4 | 23.9 | 15.5 | 6.6 | 9.5 | 5.3 | 12.1 | 100 | |
| Ant | * | -- | * | -- | -- | -- | -- | -- | -- | -- | * | -- | -- | -- | -- | * | -- | -- | * | * | * | -- | 32 | |
| Flt | 2.9 | -- | -- | -- | -- | 9.4 | 3.9 | -- | 7.8 | * | -- | 8.1 | 3.1 | -- | -- | 3.2 | 10.1 | -- | -- | 7.5 | 7.7 | 3.0 | 55 | |
| Pyr | 2.9 | 3.1 | 8.3 | 17.1 | 8.8 | 205.8 | 10.8 | 13.8 | 8.3 | 8.7 | 3.8 | 10.2 | 7.3 | 8.7 | -- | 3.8 | 7.8 | 3.1 | 10.3 | 10.2 | 3.0 | 2.1 | 95 | |
| BaA | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 9.7 | -- | -- | -- | -- | -- | -- | -- | -- | -- | 5 | |
| ΣPAHs | 13.7 | 13.2 | 15.7 | 28.2 | 27.8 | 233.0 | 28.4 | 26.7 | 29.9 | 26.7 | 10.8 | 47.6 | 34.6 | 25.6 | 5.8 | 18.4 | 42.9 | 19.5 | 18.1 | 28.4 | 17.0 | 18.4 | ||
| Rhizome | Naph | -- | 20.2 | 15.1 | n.s. | -- | 17.8 | -- | 25.9 | 17.4 | 17.6 | 11.3 | 19.0 | -- | 12.7 | n.s. | 8.3 | 15.8 | -- | 18.6 | 18.6 | 19.9 | 12.2 | 73 |
| Phe | -- | 12.5 | 10.7 | n.s. | 10.7 | * | * | 18.6 | 4.5 | 33.7 | 3.7 | 4.5 | 18.7 | 15.2 | n.s. | 8.1 | 5.6 | -- | 18.1 | 9.7 | 22.0 | 15.1 | 82 | |
| Ant | -- | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | n.s. | -- | -- | -- | -- | -- | 5.0 | -- | 5 | |
| Flt | -- | -- | -- | n.s. | -- | -- | -- | -- | -- | 0.8 | -- | -- | 0.6 | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | 9 | |
| ΣPAHs | -- | 32.7 | 25.8 | -- | 10.7 | 17.8 | -- | 44.5 | 21.9 | 52.1 | 15.0 | 23.5 | 19.3 | 27.9 | n.s. | 16.4 | 21.4 | -- | 38.1 | 28.3 | 46.9 | 25.9 | ||
| V-Sed | Naph | 4.2 | 3.6 | -- | 2.0 | 2.1 | 1.4 | 2.2 | 2.6 | 3.9 | 4.2 | 4.3 | 4.8 | 2.3 | 95.4 | n.s. | 85.7 | 104.0 | 88.8 | 2.8 | 2.9 | 0.4 | 39.4 | 91 |
| Phe | 1.5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 1.0 | -- | 8.4 | n.s. | 12.9 | 23.0 | -- | -- | 1.1 | -- | -- | 27 | |
| Flt | -- | -- | -- | -- | -- | -- | -- | 4.4 | -- | -- | -- | -- | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | 5 | |
| Pyr | -- | -- | -- | -- | -- | -- | -- | 4.3 | -- | -- | -- | -- | -- | -- | n.s. | -- | -- | -- | -- | -- | -- | -- | 5 | |
| ΣPAHs | 5.7 | 3.6 | -- | 2.0 | 2.1 | 1.4 | 2.2 | 11.3 | 3.9 | 4.2 | 4.3 | 5.8 | 2.3 | 103.8 | n.s. | 98.6 | 127.0 | 88.8 | 2.8 | 4.0 | 0.4 | 39.4 | ||
| NV-Sed | Naph | 2.1 | 3.4 | 2.1 | 2.5 | 3.9 | 1.0 | 1.6 | 4.3 | 6.3 | n.s. | 4.4 | 4.5 | 1.2 | 81.4 | n.s. | 90.0 | n.s. | n.s. | 2.3 | 0.8 | 1.3 | 79.1 | 82 |
| Phe | -- | -- | -- | -- | -- | -- | -- | 47.0 | 10.1 | n.s. | -- | -- | -- | * | n.s. | 15.5 | n.s. | n.s. | -- | -- | -- | -- | 18 | |
| Ant | -- | -- | -- | -- | -- | -- | -- | 3.0 | -- | n.s. | -- | -- | -- | -- | n.s. | -- | n.s. | n.s. | -- | -- | -- | -- | 5 | |
| Flt | -- | -- | -- | -- | -- | -- | -- | 40.7 | 13.3 | n.s. | -- | -- | -- | -- | n.s. | -- | n.s. | n.s. | -- | -- | -- | -- | 9 | |
| Pyr | -- | -- | -- | -- | -- | -- | -- | 30.8 | 11.7 | n.s. | -- | -- | -- | -- | n.s. | -- | n.s. | n.s. | -- | -- | -- | -- | 9 | |
| ΣPAHs | 2.1 | 3.4 | 2.1 | 2.5 | 3.9 | 1.0 | 1.6 | 125.8 | 41.4 | n.s. | 4.4 | 4.5 | 1.2 | 81.4 | n.s. | 106 | n.s. | n.s. | 2.3 | 0.8 | 1.3 | 79.1 | ||
| Matrix | Analyte | EE3 | RM6 | V1 | M1 | M2 | M3 | Detection | ||||||||||||||||
| NMR-Sed | Naph | 5.0 | 32.4 | 86.4 | 72.7 | 70.7 | 79.0 | 100 | ||||||||||||||||
| Flu | -- | 3.4 | -- | -- | -- | -- | 17 | |||||||||||||||||
| Phe | 3.7 | 7.5 | 4.2 | -- | -- | -- | 50 | |||||||||||||||||
| Flt | 3.9 | -- | -- | -- | -- | -- | 17 | |||||||||||||||||
| ΣPAHs | 12.6 | 43.3 | 90.6 | 72.7 | 70.7 | 79.0 |
| Matrix | Analyte | Concentration | EE2 | EE4 | RM1 | RM2 | RM3 | RM4 | RM5 | ALM1 | ALM2 | ALM3 | CG2 | CG3 | CG4 | C2 | V2 | ALI1 | ALI2 | ALI3 | ALI4 | ALI5 | ALI6 | ALI7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Pyr | Predicted | 7.2 | 7.7 | 11.8 | 25.6 | 12.5 | 223.7 | 15.3 | 20.7 | 11.8 | 12.4 | 9.5 | 14.5 | 10.3 | 12.3 | - | 9.4 | 11.0 | 7.6 | 14.7 | 14.5 | 7.3 | 5.2 |
| True | 2.9 | 3.1 | 8.3 | 17.1 | 8.8 | 205.8 | 10.8 | 13.8 | 8.3 | 8.7 | 3.8 | 10.2 | 7.3 | 8.7 | - | 3.8 | 7.8 | 3.1 | 10.3 | 10.2 | 3.0 | 2.1 | ||
| Error Factor | 2.5 | 2.5 | 1.4 | 1.5 | 1.4 | 1.1 | 1.4 | 1.5 | 1.4 | 1.4 | 2.5 | 1.4 | 1.4 | 1.4 | - | 2.5 | 1.4 | 2.5 | 1.4 | 1.4 | 2.4 | 2.5 | ||
| Rhizomes | Naph | Predicted | - | 13.8 | - | - | - | 10.6 | - | 17.4 | 12.1 | 12.2 | 8.3 | 13.1 | - | 9.1 | 12.3 | 6.4 | 11.0 | - | 12.8 | 12.8 | 13.6 | 8.8 |
| True | - | 20.2 | - | - | - | 15.1 | - | 25.9 | 17.4 | 17.6 | 11.3 | 19.0 | - | 12.7 | 17.8 | 8.3 | 15.8 | - | 18.6 | 18.6 | 19.9 | 12.2 | ||
| Error Factor | - | 1.5 | - | - | - | 1.4 | - | 1.5 | 1.4 | 1.4 | 1.4 | 1.5 | - | 1.4 | - | 1.3 | 1.4 | - | 1.5 | 1.5 | 1.5 | 1.4 | ||
| Matrix | Analyte | Concentration | EE4 ░ | EE4 ▓ | RM2 ▓ | RM5 ▓ | RM6 ▒ | ALI3 ▓ | ||||||||||||||||
| Sediment | Naph | Predicted | 4.5 | 4.7 | 3.2 | 2.8 | 2.4 | 223.3 | ||||||||||||||||
| True | 3.4 | 3.6 | 2 | 2.2 | 1.9 | 81.3 | ||||||||||||||||||
| Error Factor | 1.3 | 1.3 | 1.6 | 1.3 | 1.3 | 2.7 |
| Compound | CAS | Molecular Formula | Molecular Mass | RT ± SD | HRF (%) | Matrix |
|---|---|---|---|---|---|---|
| TBP | 126-73-8 | C12H27O4P | 266.16415 | 5.84 ± 0.01 | 100.0 | ■ |
| DBAL | 5779-94-2 | C9H10O | 133.06473 | 6.73 ± 0.00 | 87.4 | ▓ ░ ▒ |
| Isop | 2631-40-5 | C11H15NO2 | 193.10973 | 8.29 ± 0.01 | 89.2 | ◊ |
| 2,4-DTBP | 128-39-2 | C14H22O | 206.16706 | 8.89 ± 0.00 | 95.7–96.5 | ◊ ▓ ░ ▒ |
| 7,9-DTBO | 82304-66-3 | C17H24O3 | 276.17254 | 12.39 ± 0.01 | 95.8–99.9 | ◊ ▓ ░ ▒ |
| 1-MOF | 139552-06-0 | C15H14O | 210.10446 | 12.42 ± 0.01 | 90.3 | ▓ ░ ▒ |
| DAIP | 1087-21-4 | C14H14O4 | 246.08921 | 14.06 ± 0.01 | 94.1 | ◊ |
| Dodem | 1593-77-7 | C18H35NO | 281.27186 | 16.23 ± 0.01 | 97.5–97.7 | ◊ ▓ ░ ▒ |
| DCHP | 84-61-7 | C20H26O4 | 330.18311 | 17.61± 0.01 | 100.0 | ◊ |
| Irg 168 | 31570-04-4 | C42H63O3P | 646.45148 | 24.22 ± 0.00 | 92.9–97.7 | ◊ ▓ ░ ▒ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo-Pascual, M.; Tudor, R.; Domínguez, I.; Aguilera, P.A.; Frenich, A.G. Determination of PAHs, PAH-Derivatives and Other Concerning Substances in Posidonia oceanica Seagrass and Marine Sediments by High Resolution Mass Spectrometry. J. Mar. Sci. Eng. 2023, 11, 369. https://doi.org/10.3390/jmse11020369
Astudillo-Pascual M, Tudor R, Domínguez I, Aguilera PA, Frenich AG. Determination of PAHs, PAH-Derivatives and Other Concerning Substances in Posidonia oceanica Seagrass and Marine Sediments by High Resolution Mass Spectrometry. Journal of Marine Science and Engineering. 2023; 11(2):369. https://doi.org/10.3390/jmse11020369
Chicago/Turabian StyleAstudillo-Pascual, Marina, Roxana Tudor, Irene Domínguez, Pedro A. Aguilera, and Antonia Garrido Frenich. 2023. "Determination of PAHs, PAH-Derivatives and Other Concerning Substances in Posidonia oceanica Seagrass and Marine Sediments by High Resolution Mass Spectrometry" Journal of Marine Science and Engineering 11, no. 2: 369. https://doi.org/10.3390/jmse11020369