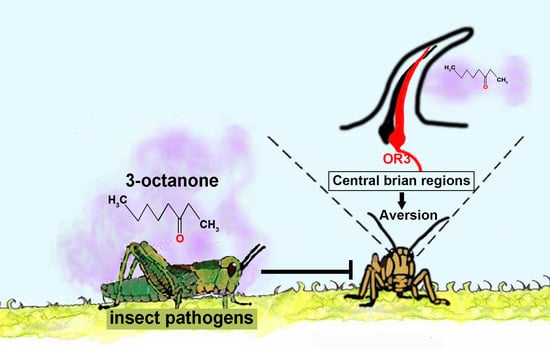
The Aversive Response of the Locust Locusta migratoria to 3-Octanone, an Odorant from Fungal Pathogens, Is Mediated by a Chemosensory Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Electroantennogram (EAG)
2.3. Whole-Mount Fluorescence In Situ Hybridization (WM-FISH)
2.4. Single Sensillum Recording (SSR)
2.5. Preference Behavioral Bioassay
2.6. RNA Interference (RNAi)
2.7. Real-Time Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. EAG Responses of Locusts to 18 Compounds at Different Concentrations
- 2-heptanone, 4, 5-dimethylthiazole, and 2, 4, 5-trimethylthiazole;
- 2, 5-dimethypyrazine;
- 3-heptanone, 2-octanone, and penthyl acetate;
- 3-octanone and butyl acetate (Figure 1C).
3.2. Responses of Neurons in One Trichoid Sensilla to 18 Compounds
3.3. Preference Behavior of Locusts in Response to 11 Compounds
3.4. LmigOR3 Is Expressed in Neuron A in the Tested Sensilla and Mediates 3-Octanone-Induced Rejection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and grasshopper management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M. Can the war on locusts be won? Science 2004, 306, 1800. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Berry, K.; Buhl, J.; De Keyser, R.; Foquet, B.; Hadrich, J.C.; Matheson, T.; et al. From molecules to management: Mechanisms and consequences of locust phase polyphenism. Adv Insect. Physiol. 2017, 53, 169–261. [Google Scholar]
- Nakano, M.; Morgan-Richards, M.; Trewick, S.A.; Clavijo-McCormick, A. Chemical Ecology and Olfaction in Short-Horned Grasshoppers (Orthoptera: Acrididae). J. Chem. Ecol. 2022, 48, 121–140. [Google Scholar] [PubMed]
- Pan, X.Q.; Liu, J.; Xu, X.; Zhang, L.W.; Zhang, L. Combinatorial olfactory signaling in short-distance determines host plant recognition in locust. Agriculture 2023, 13, 1030. [Google Scholar] [CrossRef]
- Guo, X.J.; Yu, Q.Q.; Chen, D.F.; Wei, J.N.; Yang, P.C.; Yu, J.; Wang, X.H.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Wei, J.N.; Shao, W.B.; Cao, M.M.; Ge, J.; Yang, P.C.; Chen, L.; Wang, X.H.; Kang, L. Phenylacetonitrile in locusts facilitates an antipredator defense by acting as an olfactory aposematic signal and cyanide precursor. Sci. Adv. 2019, 5, eaav5495. [Google Scholar]
- Chang, H.T.; Cassau, S.; Krieger, J.; Guo, X.J.; Knaden, M.; Kang, L.; Hansson, B.S. A chemical defense deters cannibalism in migratory locusts. Science 2023, 380, 537–543. [Google Scholar] [CrossRef]
- Rath, C.A. The use of entomopathogenic fungi for control of termites. Biocontrol Sci. Technol. 2000, 10, 563–581. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Gerardo, N.M.; Inouye, D.W.; Medina, M.; Xavier, J.B. Animal behavior and the microbiome. Science 2012, 338, 198–199. [Google Scholar] [CrossRef]
- Meyling, N.V.; Pell, J.K. Detection and avoidance of entomopathogenic fungi by a generalist insect predator. Ecol. Entomol. 2006, 31, 162–171. [Google Scholar] [CrossRef]
- Mburu, D.M.; Ochola, L.; Maniania, N.K.; Njagi, P.G.N.; Gitonga, L.M.; Ndung’u, M.W.; Wanjoya, A.K.; Hassanali, A. Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J. Insect Physiol. 2009, 55, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Mattheis, J.P.; Roberts, R.G. Identification of geosmin as a volatile metabolite of Penicillium expansum. Appl. Environ. Microbiol. 1992, 58, 3170–3172. [Google Scholar] [CrossRef] [PubMed]
- Gerber, N.N.; Lechevalier, H.A. Geosmin, an earthly-smelling substance isolated from actinomycetes. Appl. Microbiol. 1965, 13, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar]
- Stensmyr, M.A.; Dweck, H.K.M.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A Conserved Dedicated Olfactory Circuit for Detecting Harmful Microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Getchell, T.V.; Margolis, F.L.; Getchell, M.L. Perireceptor and receptor events in vertebrate olfaction. Prog Neurobiol. 1984, 23, 317–345. [Google Scholar] [CrossRef]
- Hallem, E.A.; Ho, M.G.; Carlson, J.R. The molecular basis of odor coding in the Drosophila antenna. Cell 2004, 117, 965–979. [Google Scholar] [CrossRef] [Green Version]
- Carey, F.A.; Wang, G.R.; Su, C.Y.; Zwiebel, L.J.; Carlson, J.R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 2010, 464, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Speth, Z.; Kaur, K.; Mazolewski, D.; Sisomphou, R.; Siao, D.D.C.; Pooraiiouby, R.; Vasquez-Gross, H.; Petereit, J.; Gulia-Nuss, M.; Mathew, D.; et al. Characterization of Anopheles stephensi Odorant Receptor 8, an Abundant Component of the Mouthpart Chemosensory Transcriptome. Insects 2021, 12, 593. [Google Scholar] [CrossRef]
- Chang, H.T.; Unni, A.; Tom, M.T.; Llorca, L.C.; Brase, S.; Bucks, S.; Weniger, K.; Bisch-Knaden, S.; Hansson, B.S.; Knaden, M. Non-redundant odorant detection in a locust. bioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Syed, Z.; Ishida, Y.; Taylor, K.; Kimbrell, D.A.; Leal, W.S. Pheromone reception in fruit flies expressing a moth’s odorant receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 16538–16543. [Google Scholar] [CrossRef] [PubMed]
- You, Y.W.; Smith, D.P.; Lv, M.Y.; Zhang, L. A broadly tuned odorant receptor in neurons of trichoid sensilla in locust, Locusta migratoria. Insect Biochem Molec. 2016, 79, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.Z.; Guo, M.; Yang, Y.; Zhang, L. Differential expression of two odorant receptors in Locusta migratoria. BMC Neurosci. 2013, 14, 50. [Google Scholar] [CrossRef] [Green Version]
- Li, H.W.; Wang, P.; Zhang, L.W.; Xu, X.; Cao, Z.W.; Zhang, L. Expressions of Olfactory Proteins in Locust Olfactory Organs and a Palp Odorant Receptor Involved in Plant Aldehydes Detection. Front. Physiol. 2018, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Schultze, A.; Pregitzer, P.; Walter, M.F.; Woods, D.F.; Marinotti, O.; Breer, H.; Krieger, J. The co-expression pattern of odorant binding proteins and olfactory receptors identify distinct trichoid sensilla on the antenna of the malaria mosquito Anopheles gambiae. PLoS ONE 2013, 8, e69412. [Google Scholar] [CrossRef]
- Xu, X.; You, Y.W.; Zhang, L. Localization of odorant receptor genes in locust antennae by RNA in situ hybridization. Jove 2017, 125, e55924. [Google Scholar]
- Cui, X.J.; Wu, C.H.; Zhang, L. Electrophysiological response patterns of 16 olfactory neurons from the trichoid sensilla to odorant from fecal volatiles in the locust, Locusta migratoria manilensis. Arch. Insect. Biochem. 2011, 77, 45–57. [Google Scholar] [CrossRef]
- Li, H.W.; You, Y.W.; Zhang, L. Single sensillum recordings for locust palp sensilla basiconica. Jove-J. Vis. Exp. 2018, 136, 57863. [Google Scholar] [CrossRef] [Green Version]
- Obengofori, D.; Torto, B.; Hassanali, A. Evidence for mediation of two releaser pheromones in the aggregation behavior of the gregarious desert locust, Schistocerca gregaria (Forskal) (Orthoptera: Acrididae). J. Chem. Ecol. 1993, 19, 1665–1676. [Google Scholar] [CrossRef]
- Bruyne, M.D.; Foster, K.; Carlson, J.R. Odor Coding in the Drosophila Antenna. Neuron 2001, 30, 537–552. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yin, X.W.; Zhang, L. Plant Approach-Avoidance Response in Locusts Driven by Plant Volatile Sensing at Different Ranges. J. Chem. Ecol. 2019, 45, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.H.; Yang, D.; Wu, W.; Zeng, X.K.; Jing, B.Y.; Li, M.T.; Qin, S.S.; Tang, C.; Tu, Y.H.; Luo, D.G. Odor-evoked inhibition of olfactory sensory neurons drives olfactory perception in Drosophila. Nat. Commun. 2017, 8, 1357. [Google Scholar] [CrossRef] [Green Version]
- Su, C.Y.; Menuz, K.; Reisert, J.; Carlson1, J.R. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 2012, 492, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Bhandawat, V.; Olsen, S.R.; Gouwens, N.W.; Schlief, M.L.; Wilson, R.I. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 2007, 10, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.R.; Bhandawat, V.; Wilson, R.I. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 2007, 54, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herre, M.; Goldman, O.V.; Lu, T.C.; Caballero-Vidal, G.; Qi, Y.Y.; Gilbert, Z.N.; Gong, Z.Y.; Morita, T.; Rahiel, S.; Ghaninia, M.; et al. Non-canonical odor coding in the mosquito. Cell 2022, 185, 3104–3123. [Google Scholar] [CrossRef]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Gołębiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, A.; Imai, T.; Akino, T.; Toh, Y.; Yoshimura, T. Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus. J. Chem. Ecol. 2015, 41, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol. Control 2021, 152, 104472. [Google Scholar] [CrossRef]
- Hummadi, E.H.; Dearden, A.; Generalovic, T.; Clunie, B.; Harrott, A.; Cetin, Y.; Demirbek, M.; Khoja, S.; Eastwood, D.; Dudley, E.; et al. Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol. Control 2021, 155, 104527. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.O.H.; Howse, P.E.; Vilela, E.F.; Goulson, D. The response of grass-cutting ants to natural and synthetic versions of their alarm pheromone. Physiol. Entomol. 2001, 26, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Yang, M.Y.; Ma, B.W.; Zhao, Y.; Zhuang, H.N.; Zhang, J.S.; Chen, D. Volatile profiles of two genotype Agaricus bisporus species at different growth stages. Food Res. Int. 2021, 140, 109761. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, L.; Zhao, X. The Aversive Response of the Locust Locusta migratoria to 3-Octanone, an Odorant from Fungal Pathogens, Is Mediated by a Chemosensory Protein. Agriculture 2023, 13, 1542. https://doi.org/10.3390/agriculture13081542
Xu X, Zhang L, Zhao X. The Aversive Response of the Locust Locusta migratoria to 3-Octanone, an Odorant from Fungal Pathogens, Is Mediated by a Chemosensory Protein. Agriculture. 2023; 13(8):1542. https://doi.org/10.3390/agriculture13081542
Chicago/Turabian StyleXu, Xiao, Long Zhang, and Xingbo Zhao. 2023. "The Aversive Response of the Locust Locusta migratoria to 3-Octanone, an Odorant from Fungal Pathogens, Is Mediated by a Chemosensory Protein" Agriculture 13, no. 8: 1542. https://doi.org/10.3390/agriculture13081542