The Preharvest Application of Essential Oils (Carvacrol, Eugenol, and Thymol) Reduces Fungal Decay in Lemons
Abstract
:1. Introduction
2. Materials and Methods
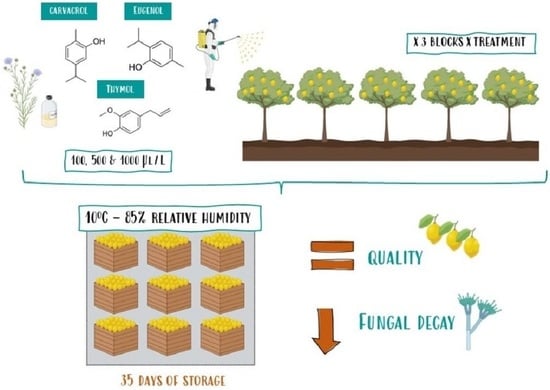
2.1. Plant Material and Experimental Design
2.2. Decay Incidence of Lemons
2.3. Physiological and Quality Parameters
2.4. Total Phenolic Content
2.5. Statistical Analysis
3. Results
3.1. Effect of EOs in the Decay Incidence of Lemons
3.2. Effect of EOs in the Quality Parameters of ‘Fino’ and ‘Verna’ Lemons

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, B.L.; Nelson, R.G.; Ebel, R.C.; Dozier, W.A.; Adrian, J.L.; Hockema, B.R. Fruit quality characteristics that affect consumer preferences for Satsuma mandarins. HortScience 2004, 39, 1664–1669. [Google Scholar] [CrossRef]
- FAOSTAT. Production Statistics. 2021. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 5 June 2023).
- Strano, M.C.; Altieri, G.; Admane, N.; Genovese, F.; Di Renzo, G.C. Advance in citrus postharvest management: Diseases, cold storage and quality evaluation. In Citrus Pathology; InTechOpen: London, UK, 2017; pp. 139–159. [Google Scholar] [CrossRef] [Green Version]
- Palou, L. Penicillium digitatum, Penicillium italicum (Green Mold, Blue Mold). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 45–102. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Gabler, F.M.; Sorenson, D. Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Technol. 2008, 47, 226–238. [Google Scholar] [CrossRef]
- Altieri, G.; Di Renzo, G.C.; Genovese, F.; Calandra, M.; Strano, M.C. A new method for the postharvest application of imazalil fungicide to citrus fruit. Biosyst. Eng. 2013, 115, 434–443. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Prelle, A.; Garibaldi, A.; Gullino, M.L. Efficacy of plant essential oils on post-harvest control of rots caused by fungi on different stone fruits In Vivo. J. Food Prot. 2013, 76, 631–639. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; OuYang, Q.; Duan, B.; Wang, Z.; Meng, K.; Tan, X.; Tao, N. γ-Cyclodextrin encapsulated thymol for citrus preservation and its possible mechanism against Penicillium digitatum. Pestic. Biochem. Physiol. 2023, 194, 105501. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Ma, X.; Jia, C.; Han, J.; Song, C.; Liu, Y.; Wei, D.; Xu, H.; Qin, J.; et al. Nano-emulsification essential oil of Monarda didyma L. to improve its preservation effect on postharvest blueberry. Food Chem. 2023, 417, 135880. [Google Scholar] [CrossRef]
- Arras, G.; Usai, M. Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: Chemical analysis of Thymus capitatus oil and its effect in subatmospheric pressure conditions. J. Food Prot. 2001, 64, 1025–1029. [Google Scholar] [CrossRef]
- Ameziane, N.; Boubaker, H.; Boudyach, H.; Msanda, F.; Jilal, A.; Ait Benaoumar, A. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 2007, 27, 273–277. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.P.; Li, Y.C.; Li, H.Y.; Yu, T.; Zheng, X.D. Antifungal activity of Thyme oil against Geotrichum citri-aurantii in Vitro and In Vivo. J. Appl. Microbiol. 2009, 107, 1450–1456. [Google Scholar] [CrossRef]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In Vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crops Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of Citrus. Ind. Crops Prod. 2014, 61, 151–159. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of Citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol potential of essential oils in organic horticulture systems: From farm to fork. Front. Nutr. 2022, 8, 805138. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alfonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Serrano, M.; Valero, D.; Rodríguez-López, M.I.; Gabaldón, J.A.; Castillo, S.; Valverde, J.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D. Thymol encapsulated into HP-β-Cyclodextrin as an alternative to synthetic fungicides to induce lemon resistance against sour rot decay. Molecules 2020, 25, 4348. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Bailén, G.; Zapata, P.; Serrano, M.; Castillo, S.; Valero, D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int. J. Food Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef]
- Martínez, J.A.; González, R. Essential oils from clove affect growth of Penicillium species obtained from lemons. Commun. Agric. Appl. Biol. Sci. 2013, 78, 563–572. [Google Scholar] [PubMed]
- Álvarez, M.V.; Palou, L.; Taberner, V.; Fernández-Catalán, A.; Argente-Sanchis, M.; Pitta, E.; Pérez-Gago, M.B. Natural pectin-based edible composite coatings with antifungal properties to control green mold and reduce losses of ‘Valencia’ oranges. Foods 2022, 11, 1083. [Google Scholar] [CrossRef]
- Cháfer, M.; Sánchez-González, L.; González-Martínez, C.; Chiralt, A. Fungal decay and shelf life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J. Food Sci. 2012, 77, E182–E187. [Google Scholar] [CrossRef] [Green Version]
- Ramezanian, A.; Azadi, M.; Mostowfizadeh-Ghalamfarsa, R.; Saharkhiz, M.J. Effect of Zataria multiflora Boiss and Thymus vulgaris L. essential oils on black rot of “Washington Navel” orange fruit. Postharvest Biol. Technol. 2016, 112, 152–158. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Chen, X.; Chen, C.; Simal-Gandara, J.; Chen, J.; Wan, C. Essential oils nano-emulsion confers resistance against Penicillium digitatum in “Newhall” navel orange by promoting phenylpropanoid metabolism. Ind. Crops Prod. 2022, 187, 115297. [Google Scholar] [CrossRef]
- Zapata, P.J.; Díaz-Mula, H.M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. The combination of alginate coating and essential oils delayed postharvest ripening and increased the antioxidant potential of two sweet cherries. Acta Hortic. 2017, 1161, 633–638. [Google Scholar] [CrossRef]
- Castillo, S.; Pérez-Alfonso, C.O.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control 2014, 35, 132–136. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-Vitro antimicrobial activity and chemical composition of sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Hosseini, S.; Amini, J.; Rafei, J.N.; Khorshidi, J. Management of strawberry anthracnose using plant essential oils as biofungicides, and evaluation of their effects on quality of strawberry fruit. J. Oleo Sci. 2020, 69, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Migliori, C.A.; Salvati, L.; Di Cesare, L.F.; Lo Scalzo, R.; Parisi, M. Effects of preharvest applications of natural antimicrobial products on tomato fruit decay and quality during long-term storage. Sci. Hortic. 2017, 222, 193–202. [Google Scholar] [CrossRef]
- Black-Solis, J.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.; Corona-Rangel, M.L.; Bautista-Baños, S. Pre-harvest use of biodegradable polyester nets added with cinnamon essential oil and the effect on the storage life of tomatoes and the development of Alternaria alternata. Sci. Hortic. 2019, 245, 65–73. [Google Scholar] [CrossRef]
- Goñi, M.G.; Tomadoni, B.; Moreira, M.R.; Roura, S.I. Application of Tea Tree and Clove Essential Oil on Late Development Stages of Butterhead Lettuce: Impact on Microbiological Quality. LWT-Food Sci. Technol. 2013, 54, 107–113. [Google Scholar] [CrossRef]
- Goñi, M.G.; Tomadoni, B.; Roura, S.I.; Moreira, M.R. Effect of preharvest application of chitosan and tea tree essential oil on postharvest evolution of lettuce native microflora and exogenous Escherichia coli O157:H7. J. Food Saf. 2014, 34, 353–360. [Google Scholar] [CrossRef]
- Viacava, G.E.; Goyeneche, R.; Goñi, M.G.; Roura, S.I.; Agüero, M.V. Natural elicitors as preharvest treatments to improve postharvest quality of butterhead lettuce. Sci. Hortic. 2018, 228, 145–152. [Google Scholar] [CrossRef]
- Hosseini, S.; Amini, J.; Saba, M.K.; Karimi, K.; Pertot, I. Preharvest and postharvest application of garlic and rosemary essential oils for controlling anthracnose and quality assessment of strawberry fruit during cold storage. Front. Microbiol. 2020, 11, 1855. [Google Scholar] [CrossRef] [PubMed]
- Rajestary, R.; Xylia, P.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Preharvest application of commercial products based on chitosan, phosphoric acid plus micronutrients, and orange essential oil on postharvest quality and gray mold infections of strawberry. Int. J. Mol. Sci. 2022, 23, 15472. [Google Scholar] [CrossRef]
- Zapata, P.J.; Castillo, S.; Valero, D.; Guillén, F.; Serrano, M.; Díaz-Mula, H.M. The Use of alginate as edible coating alone or in combination with essential oils maintained postharvest quality of tomato. Acta Hortic. 2010, 877, 1529–1534. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest treatment with oxalic acid improves postharvest storage of lemon fruit by stimulation of the antioxidant system and phenolic content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Pre-harvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Mar-tínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Guillén, F.; Zapata, P.J.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Improvement of the overall quality of table grapes stored under modified atmosphere packaging in combination with natural antimicrobial compounds. J. Food Sci. 2007, 72, S185–S190. [Google Scholar] [CrossRef] [PubMed]
- Mattheis, J.P.; Roberts, R. Fumigation of sweet cherry (Prunus avium ‘Bing’) fruit with low molecular weight aldehydes for postharvest decay control. Plant Dis. 1993, 77, 810–814. [Google Scholar] [CrossRef]
- Liu, W.; Chu, C.; Zhou, T. Thymol and acetic acid vapors reduce postharvest brown rot of apricots and plums. HortScience 2002, 37, 151–156. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In Vitro and in Vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wee Pheng, T.; Mustafa, M.A. Application of lemongrass oil in vapour phase for the effective control of anthracnose of “Sekaki” papaya. J. Appl. Microbiol. 2015, 118, 1456–1464. [Google Scholar] [CrossRef]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Perina, F.J.; Amaral, D.C.; Fernandes, R.S.; Labory, C.R.G.; Teixeira, G.A.; Alves, E. Thymus vulgaris essential oil and thymol against Alternaria alternata (Fr.) Keissler: Effects on growth, viability, early infection and cellular mode of action. Pest Manag. Sci. 2015, 71, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, H.; Zhang, Y.; Huang, Y.; Wang, L.; Zheng, Y. UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Sci. Hortic. 2017, 225, 106–111. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against Anthracnose during Post-Harvest Storage. Crop. Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef] [Green Version]
- La Torre, A.; Caradonia, F.; Matere, A.; Battaglia, V. Using plant essential oils to control Fusarium wilt in tomato plants. Eur. J. Plant Pathol. 2016, 144, 487–496. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439802670. [Google Scholar]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef]



| Cultivar | Weight Loss | Firmness | Hue Angle | Total Soluble Solids | Titratable Acidity | Total Phenolic Content |
|---|---|---|---|---|---|---|
| ‘Fino’ cultivar | ||||||
| Time | - | 36.82 *** | ns | 155.06 *** | 4.00 ** | 3.94 * |
| Treatment | ns | ns | ns | 17.48 *** | 4.58 *** | 41.84 *** |
| Interaction Time × Treatment | - | ns | ns | 8.78 *** | ns | 4.00 *** |
| ‘Verna’ cultivar | ||||||
| Time | - | 22.66 *** | 65.56 *** | 90.82 *** | ns | 8.25 *** |
| Treatment | ns | ns | 2.03 * | 8.87 *** | ns | 3.64 ** |
| Interaction Time × Treatment | - | ns | ns | 5.91 ** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Pozo, M.; Serna-Escolano, V.; Giménez-Berenguer, M.; Giménez, M.J.; Zapata, P.J. The Preharvest Application of Essential Oils (Carvacrol, Eugenol, and Thymol) Reduces Fungal Decay in Lemons. Agriculture 2023, 13, 1437. https://doi.org/10.3390/agriculture13071437
Gutiérrez-Pozo M, Serna-Escolano V, Giménez-Berenguer M, Giménez MJ, Zapata PJ. The Preharvest Application of Essential Oils (Carvacrol, Eugenol, and Thymol) Reduces Fungal Decay in Lemons. Agriculture. 2023; 13(7):1437. https://doi.org/10.3390/agriculture13071437
Chicago/Turabian StyleGutiérrez-Pozo, María, Vicente Serna-Escolano, Marina Giménez-Berenguer, Maria J. Giménez, and Pedro J. Zapata. 2023. "The Preharvest Application of Essential Oils (Carvacrol, Eugenol, and Thymol) Reduces Fungal Decay in Lemons" Agriculture 13, no. 7: 1437. https://doi.org/10.3390/agriculture13071437