Effect of Water Deficit on Growth and Photoassimilate Partitioning in Leersia hexandra and Luziola peruviana
Abstract
:1. Introduction
2. Material and Methods
2.1. Experiments 1 and 2—Effect of Soil Water Content on Weed Growth
2.2. Experiments 3 and 4—Effect of Soil Water Content on Carbohydrate Partitioning
2.3. Carbohydrate Extraction
2.4. Total Soluble Sugar Quantification
2.5. Starch Quantification
2.6. Sucrose Quantification
2.7. Data Analysis
3. Results and Discussion
3.1. Effect of Soil Water Content on Plant Growth
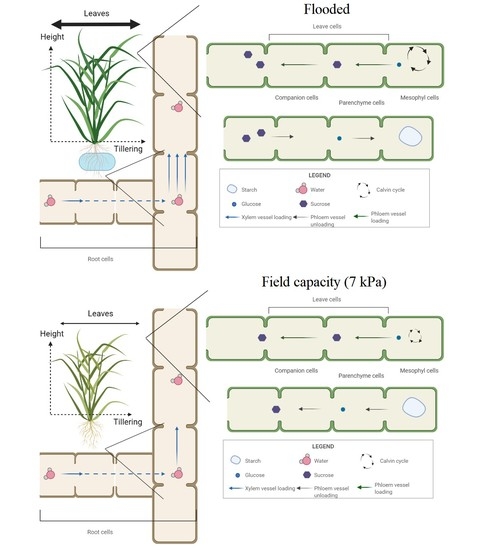
3.2. Effect of Soil Drainage on Plant Photoassimilate Partitioning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziska, L.H.; Gealy, D.R.; Burgos, N.; Caicedo, A.L.; Gressel, J.; Lawton-Rauh, A.L.; Avila, L.A.; Theisen, G.; Norsworthy, J.; Ferrero, A.; et al. Weedy (Red) Rice. An Emerging Constraint to Global Rice Production. Adv. Agron. 2015, 129, 181–228. [Google Scholar] [CrossRef]
- Villa, S.C.C.; Marchezan, E.; Massoni, P.F.S.; Santos, F.M.; Avila, L.A.; Machado, S.L.O.; Telo, G.M. Controle de arroz-vermelho em dois genótipos de arroz (Oryza sativa) tolerantes a herbicidas do grupo das imidazolinonas. Planta Daninha 2006, 24, 549–555. [Google Scholar] [CrossRef]
- Menezes, V.G.; Mariot, C.H.P.; Kalsing, A.; Freitas, T.F.S.D.; Grohs, D.S.; Matzenbacher, F.D.O. Associação de glyphosate e imidazolinonas no controle de arroz-vermelho em arroz Clearfield. Ciência Rural 2013, 43, 2154–2159. [Google Scholar] [CrossRef]
- Galon, L.; Agostinetto, D.; Moraes, P.V.D.; Tironi, S.P.; Dal Magro, T. Estimativa das perdas de produtividade de grãos em cultivares de arroz (Oryza sativa) pela interferência do capim-arroz (Echinochloa spp.). Planta Daninha 2007, 25, 697–707. [Google Scholar] [CrossRef]
- Agostinetto, D.; Galon, L.; Moraes, P.V.D.; Rigoli, R.P.; Tironi, S.P.; Panozzo, L.E. Competitividade relativa entre cultivares de arroz irrigado e biótipo de Capim-Arroz (Echinochloa spp.). Planta Daninha 2008, 26, 757–766. [Google Scholar] [CrossRef]
- Pacanoski, Z.; Glatkova, G. The Use of Herbicides for Weed Control in Direct Wet-Seeded Rice (Oryza sativa L.) in Rice Production Regions in the Republic of Macedonia. Plant Protection Science. Plant Protect. Sci. 2009, 45, 113–118. [Google Scholar] [CrossRef]
- Ulguim, A.R.; Fruet, B.L.; Merotto Junior, A.; Silva, A.L. Status of Weed Control in Imidazolinone-Herbicide Resistant Rice in Rio Grande Do Sul. Adv. Weed Sci. 2021, 39. [Google Scholar] [CrossRef]
- IRGA: Instituto Rio Grandense de Arroz. Censo da Lavoura Orizícola. 2021. Available online: https://irga.rs.gov.br/upload/arquivos/202109/27151231-boletim-de-resultados-da-safra-2020-2021-compressed.pdf (accessed on 20 May 2022).
- Scherner, A.; Avila, L.A.D.; Schreiber, F.; Kruse, N.D.; Agostinetto, D.; Pinto, J.J.O.; Pestana, R.R. Suscetibilidade de duas Gramas-boiadeiras a diferentes formulações de glyphosate. Ciência Rural 2014, 44, 400–406. [Google Scholar] [CrossRef]
- Scherner, A.; Avila, L.; Schreiber, F.; Kruse, N.D.; Fernando, J.A.; Garcia, É.N. Susceptibility of Peruvian Watergrass and Rice Cutgrass to Glyphosate under Soil Moisture Variations. Crop Prot. 2017, 98, 1–7. [Google Scholar] [CrossRef]
- Marchezan, M.G.; Avila, L.A.; Schaedler, C.E.; Amarante, L.; Scherner, A.; Schreiber, F. Alterações Bioquímicas de Plantas Daninhas Em Resposta Ao Estresse Causado Por Herbicidas e Submersão Total de Plantas. Planta Daninha 2017, 35. [Google Scholar] [CrossRef]
- Amato, C.G.; Sponchiado, M.; Schwarzbold, A. Estrutura de uma Comunidade de Macrófitas Aquáticas em um Açude de Contenção (São Jerônimo, RS). Rev. Bras. Biociências 2007, 5, 828–830. [Google Scholar]
- Senseman, S.A.; Armbrust, K. Weed Science Society of America. Herbicide Handbook; Weed Science Society of America: Lawrence, MA, USA, 2007; ISBN 1891276565. [Google Scholar]
- Singer, S.D.; Zou, J.; Westlake, R.J. Abiotic Factors Influence Plant Storage Lipid Accumulation and Composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, T.F.; da Cunha, F.F.; da Silva, G.H.; Condé, S.B.; Silva, F.C.D.S. Water Use of Different Weed Species Using Lysimeter and NDVI. Adv. Weed Sci. 2021, 39, e021233767. [Google Scholar] [CrossRef]
- Le, D.; Morell, M. Influence of Water Regimes and Herbicides for Control Purple Nutsedge (Cyperus rotundus). Adv. Weed Sci. 2021, 39, e20210085. [Google Scholar] [CrossRef]
- Rodrigues, J.; Inzé, D.; Nelissen, H.; Saibo, N.J.M. Source–Sink Regulation in Crops under Water Deficit. Trends Plant Sci. 2019, 24, 652–663. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The Metabolic Response to Drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Imadi, S.R.; Gul, A.; Dikilitas, M.; Karakas, S.; Sharma, I.; Ahmad, P. Water Stress: Types, Causes, and Impact on Plant Growth and Development. In Water Stress and Crop Plants: A Sustainable Approach; Wiley: Hoboken, NJ, USA, 2016; Volume 2, pp. 343–355. ISBN 9781119054450. [Google Scholar]
- Barbat Parfitt, J.M.; Andres, A.; Concenço, G.; Teló, G.M.; Schreiber, F.; da Silva, J.T.; Moisinho, I.S.; Timm, P.A. Rice and Barnyardgrass: Water Stress and Initial Establishment. Am. J. Plant Sci. 2017, 08, 3110–3119. [Google Scholar] [CrossRef]
- Graham, D.; Smydzuk, J. Use of Anthrone in the Quantitative Determination of Hexose Phosphates. Anal. Biochem. 1965, 11, 246–255. [Google Scholar] [CrossRef]
- Van Handel, E. Direct Microdetermination of Sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.5; R Foundation for Statistical Computing: Vienna, Austria, 2021; p. 55. ISBN 3-900051-07-0. Available online: https://www.r-project.org/ (accessed on 1 December 2021).
- Wickham, H. Ggplot2—Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2017; 260p, ISBN 978-3-319-24275-0. [Google Scholar]
- Lee, B.R.; Kim, K.Y.; Jung, W.J.; Avice, J.C.; Ourry, A.; Kim, T.H. Peroxidases and Lignification in Relation to the Intensity of Water-Deficit Stress in White Clover (Trifolium Repens L.). J. Exp. Bot. 2007, 58, 1271–1279. [Google Scholar] [CrossRef]
- Borrajo, C.I.; Sánchez-Moreiras, A.M.; Reigosa, M.J. Morpho-Physiological Responses of Tall Wheatgrass Populations to Different Levels of Water Stress. PLoS ONE 2018, 13, e0209281. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Varone, L.; Crescente, M.F.; Catoni, R.; Ricotta, C.; Puglielli, G. Leaf Thickness, and Density Drive the Responsiveness of Photosynthesis to Air Temperature in Mediterranean Species according to Their Leaf Habitus. J. Arid Environ. 2018, 150, 9–14. [Google Scholar] [CrossRef]
- Alderotti, F.; Brunetti, C.; Marino, G.; Centritto, M.; Ferrini, F.; Giordano, C.; Tattini, M.; Moura, B.B.; Gori, A. Coordination of Morpho-Physiological and Metabolic Traits of Cistus incanus L. to Overcome Heatwave-Associated Summer Drought: A Two-Year on-Site Field Study. Front. Ecol. Evol. 2020, 8, 576296. [Google Scholar] [CrossRef]
- Brunetti, C.; Loreto, F.; Ferrini, F.; Gori, A.; Guidi, L.; Remorini, D.; Centritto, M.; Fini, A.; Tattini, M. Metabolic Plasticity in the Hygrophyte Moringa Oleifera Exposed to Water Stress. Tree Physiol. 2018, 38, 1640–1654. [Google Scholar] [CrossRef]
- Pic, E.; Teyssendier de la Serve, B.; Tardieu, F.; Turc, O. Leaf Senescence Induced by Mild Water Deficit Follows the Same Sequence of Macroscopic, Biochemical, and Molecular Events as Monocarpic Senescence in Pea. Plant Physiol. 2002, 128, 236–246. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of Drought and Heat Stresses on Plant Growth and Yield: A Review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Q.; Zhang, Z.; Jiao, X.; Song, X.; Li, J. Vapour Pressure Deficit Control in Relation to Water Transport and Water Productivity in Greenhouse Tomato Production during Summer. Sci. Rep. 2017, 7, 43461. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wang, Z.; Niu, J. Effects of Water Stress on Fluorescence Parameters and Photosynthetic Characteristics of Drip Irrigation in Rice. Water 2020, 12, 289. [Google Scholar] [CrossRef]
- Alou, I.N.; Steyn, J.M.; Annandale, J.G.; van der Laan, M. Growth, Phenological, and Yield Response of Upland Rice (Oryza sativa L. Cv. Nerica 4®) to Water Stress during Different Growth Stages. Agric. Water Manag. 2018, 198, 39–52. [Google Scholar] [CrossRef]
- Mauad, M.; Crusciol, C.A.C.; Filho, H.G. Produção de Massa Seca e Nutrição de Cultivares de Arroz de Terras Altas Sob Condição de Déficit Hídrico e Adubação Silicatada. Semin. Cienc. Agrárias 2011, 32, 939–948. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of Water Stress on Processing Tomatoes Yield, Quality and Water Use Efficiency with Plastic Mulched Drip Irrigation in Sandy Soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, J.; Chen, Y.; Zhao, Y.; Kong, Q.; Lang, Y. Effects of Extreme Soil Water Stress on Photosynthetic Efficiency and Water Consumption Characteristics of Tamarix Chinensis in China’s Yellow River Delta. J. For. Res. 2017, 28, 491–501. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, J.; Kataria, S.; Verma, S.K.; Chatterjee, S.; Jain, M.; Pathak, K.; Rastogi, A.; Brestic, M. Regulation of the Calvin Cycle under Abiotic Stresses: An Overview. In Plant Life Under Changing Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 681–717. [Google Scholar]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020; 659p, ISBN 9780128191477. [Google Scholar]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon Source–Sink Relationship in Arabidopsis Thaliana: The Role of Sucrose Transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- Traversari, S.; Francini, A.; Laura Traversi, M.; Emiliani, G.; Sorce, C.; Sebastiani, L.; Giovannelli, A.; Laura, M. Can Sugar Metabolism in the Cambial Region Explain the Water Deficit Tolerance in Poplar? Running Title: Sugar Metabolism Regulates Water Deficit Tolerance in Poplar. J. Exp. Bot. 2018, 69, 4083–4097. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of Various Drought Stresses and Subsequent Recovery on Proline, Total Soluble Sugar and Starch Metabolisms in Rice (Oryza Sativa L.) Varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.S.; Scherner, A.; Da-Silva, C.J.; Camargo, E.R.; Palacios-Zuñiga, R.M.; Schreiber, F.; Amarante, L.d.; Parfitt, J.M.B.; Avila, L.A.d. Effect of Water Deficit on Growth and Photoassimilate Partitioning in Leersia hexandra and Luziola peruviana. Agriculture 2022, 12, 1261. https://doi.org/10.3390/agriculture12081261
Silva RS, Scherner A, Da-Silva CJ, Camargo ER, Palacios-Zuñiga RM, Schreiber F, Amarante Ld, Parfitt JMB, Avila LAd. Effect of Water Deficit on Growth and Photoassimilate Partitioning in Leersia hexandra and Luziola peruviana. Agriculture. 2022; 12(8):1261. https://doi.org/10.3390/agriculture12081261
Chicago/Turabian StyleSilva, Renan Souza, Ananda Scherner, Cristiane Jovelina Da-Silva, Edinalvo Rabaioli Camargo, Roque Mauricio Palacios-Zuñiga, Fabio Schreiber, Luciano do Amarante, José Maria Barbat Parfitt, and Luis Antonio de Avila. 2022. "Effect of Water Deficit on Growth and Photoassimilate Partitioning in Leersia hexandra and Luziola peruviana" Agriculture 12, no. 8: 1261. https://doi.org/10.3390/agriculture12081261