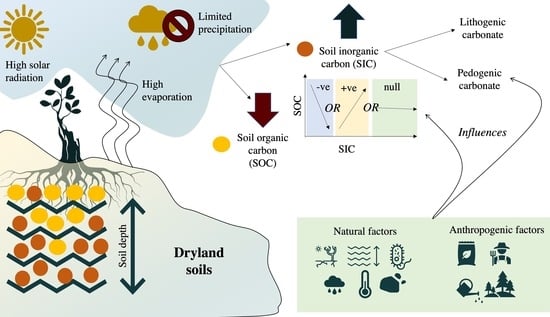
Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils—A Review
Abstract
:1. Introduction
2. Methodological Approach
3. Distribution of Arid Soils and Their Soil Constraints
4. Mechanism of SIC Formation in Dryland Areas
- (a)
- The Per Descendum model: The dissolved carbonate from the upper profile leach down through the soil profile and re-precipitate in the subsoil [41].
- (b)
- The Per Ascendum model: Ca2+ rises from the shallow water table through capillary movement and forms carbonates [42].
- (c)
- The In situ model: The dissolution of carbonates is followed by re-precipitation near the bedrock [42].
- (d)
- The Biogenic model: Secondary carbonates are formed through the activities of soil flora and fauna [32].
- (e)
- Complex mechanisms: All the above-given mechanisms work simultaneously or in a sequential manner based on the prevailing environmental conditions [43].
5. SIC and C Sequestration in Dryland Soils
6. Factors Affecting SIC Formation in Arid Soils
6.1. Climatic Factors
6.2. Land Cover and Land Use
6.3. Farm Management Practices
6.4. Irrigation
6.5. Soil Acidification through Fertilizers
- (a)
- Equations (3) and (4) depict the release of proton ions through nitrification and soil organic matter decomposition, respectively [70].
- (b)
- Equations (5) and (6) depict the consumption of H+ ions during the dissolution of SIC, thus releasing CO2 [94].
- (c)
- Leaching of dissolved inorganic C to groundwater [95].
6.6. Temperature
6.7. Microbial Soil Factors
6.8. Soil Depth
6.9. Parent Material
6.10. SIC in Salt-Affected Soils
7. Relationship between SIC and SOC in Dryland Soils
8. Dissolved Inorganic Carbon (DIC) as a “Missing Sink” in C Sequestration Studies
9. Inorganic C Fluxes in Dry Land Systems: Their Role in Gaseous Ecosystem C Flux
- (a)
- In the Chihuahuan Desert, soil CO2 profiles and fluxes, as well as volumetric soil moisture and temperature, were recorded by Hammerlynck et al. [135] throughout a three-month hot and dry period in both bare interplant canopy soils and under plant canopies. The results indicated that elevated CO2 might directly affect abiotic C dynamics in the dry season. Even if temperature and precipitation have no effect on the dynamics of soil CO2 and temperature, increasing atmospheric CO2 will speed up nocturnal carbonate dissolution. This could result in more carbonate dissolution and soil uptake beneath the canopy. Increasing levels of CO2 could alter the spatial and temporal patterns of PIC development during warm, dry seasons.
- (b)
- To explore how climate influences the CO2 fluxes and C balances in soil by interacting with biotic drivers, Ball et al. [136] measured soil CO2 flux in experimental field manipulations, microcosm incubations, and across natural environmental gradients of soil moisture and found that CO2 flux in dry valley soils is driven primarily by physical factors such as soil temperature and moisture, suggesting that future climate change may alter the dry valley soil C cycle. This shows the potential for arid polar soils to absorb CO2, mostly driven by abiotic factors related to climate change.
- (c)
- Soils rich in carbonaceous parent material are associated with CO2 exchange patterns that cannot be explained by biological processes, such as asymmetric daytime outgassing or nighttime CO2 uptake during times when all vegetation is senescent. Carbonate weathering reactions cannot account for either of these events because the rates of CO2 exchange are too low. By imposing ventilation-driven CO2 outgassing in a carbonate weathering model, Roland et al. [137] showed that carbonate geochemistry is accelerated and plays a substantial role in a semi-arid ecosystem’s CO2 exchange pattern. Ventilation depletes soil CO2 during the day, disrupting carbonate equilibria and accelerating carbonate precipitation and CO2 generation. At night, ventilation stops, and CO2 levels rise steadily. Increased carbonate dissolution consumes CO2 and compensates for increased daytime precipitation. This is why only a minimal effect on worldwide carbonate weathering rates is expected.
- (d)
- Pedogenic carbonate formation occurs when the soil is warm and extremely dry, not during the mean growing season, as is commonly believed [138].
- (e)
- Roby et al. [139] investigated the ways in which soil temperature, soil moisture, and gross ecosystem photosynthesis control soil CO2 flux in semi-arid ecosystems. Including soil moisture and gross ecosystem photosynthesis in the models of soil CO2 flux can help reduce the amount of uncertainty in semi-arid ecosystem C dynamics.
- (f)
- A large carbonate pool exists in arid soils, which may contribute to surface-atmosphere CO2 exchange via a diurnal cycle of carbonate dissolution and exsolution. Abiotic processes have a significant role in the C cycle of desert soils. Soper et al. [140] demonstrate that diurnally evolving CO2 occurs in part from carbonate sources, providing a source to balance the nocturnal CO2 uptake found in arid areas and likely maintaining the system at (or close to) C equilibrium.
- (g)
- Kowalski et al. [141] tested the idea that surface-atmosphere CO2 exchanges in terrestrial ecosystems can always be explained by biological processes alone, without considering geochemical cycling by karst systems. Further, large daytime CO2 emissions during prolonged drought and plant senescence contradict ecophysiological explanations. CO2 emissions in the afternoon during the summer in a temperate pasture above an accessible cave are hard to explain biologically, but they occur at the same time as cave ventilation. These studies reveal that CO2 exchanges between the atmosphere, ecosystems, and carbonate substrates are occasionally related directly.
- (h)
- Based on isotope analysis, the SIC pool adds significantly to soil CO2 and, in turn, to the average CO2 outflow [142]. This contribution was season and location sensitive. During daily cycles, the inorganic source contributed significantly to soil CO2, with the largest levels occurring during the day in tandem with the maximum respiration rates.
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conrad, K.A.; Dalal, R.C.; Dalzell, S.A.; Allen, D.E.; Menzies, N.W. The sequestration and turnover of soil organic carbon in Subtropical Leucaena-grass pastures. Agric. Ecosyst. Environ. 2017, 248, 38–47. [Google Scholar] [CrossRef]
- Dalal, R.C.; Thornton, C.M.; Allen, D.E.; Owens, J.S.; Kopittke, P.M. Long-term land use change in Australia from native forest decreases all fractions of soil organic carbon, including resistant organic carbon, for cropping but not sown pasture. Agric. Ecosyst. Environ. 2021, 311, 107326. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.E.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Chang. Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Singh, B.; Dalal, R.C.; Dijkstra, F.A. Carbon dynamics from carbonate dissolution in Australian agricultural soils. Soil Res. 2015, 53, 144. [Google Scholar] [CrossRef]
- Lu, T.; Wang, X.; Xu, M.; Yu, Z.; Luo, Y.; Smith, P. Dynamics of pedogenic carbonate in the cropland of the North China plain: Influences of intensive cropping and salinization. Agric. Ecosyst. Environ. 2020, 292, 106820. [Google Scholar] [CrossRef]
- Eswaran, H.; Van Den, H.; Berg, P.; Reich, J. Global Soil C Resources; CRC Press (Lewis Publishers): Boca Raton, FL, USA, 1995. [Google Scholar]
- Rice, C.W. Carbon Cycle in Soils. In Encyclopedia of Soils in the Environment Science; Elsevier: Amsterdam, The Netherlands, 2004; pp. 164–170. [Google Scholar]
- Lal, R. Carbon sequestration in dryland ecosystems. Environ. Manag. 2004, 33, 528–544. [Google Scholar] [CrossRef]
- Hirmas, D.R.; Amrhein, C.; Graham, R.C. Spatial and process-based modeling of soil inorganic carbon storage in an Arid Piedmont. Geoderma 2010, 154, 486–494. [Google Scholar] [CrossRef]
- IPCC Climate Change: Mitigation of Climate Change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC Climate Change: Geneva, Switzerland, 2014.
- Trumper, K.; Ravilious, C.; Dickson, B. Carbon in Drylands: Desertification, climate change and carbon finance. In Proceedings of the A UNEP-UNDP-UNCCD Technical Note for Discussions at CRIC 7, Istanbul, Turkey, 3–14 November 2008. [Google Scholar]
- Schlesinger, W.H. Carbon storage in the caliche of Arid soils: A case study from Arizona. Soil Sci. 1982, 133, 247–255. [Google Scholar] [CrossRef]
- Sombroek, W.G.; Nachtergaele, F.O.; Hebel, A. Amounts, dynamics and sequestering of carbon in Tropical and Subtropical soils. Ambio 1993, 22, 417–426. [Google Scholar]
- Batjes, N.H. Total carbon and nitrogen in the soils of the World. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Díaz-Hernández, J.L. Is soil carbon storage underestimated? Chemosphere 2010, 80, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Managing soils for resolving the conflict between agriculture and nature: The hard talk. Eur. J. Soil Sci. 2020, 71, 1–9. [Google Scholar] [CrossRef]
- Stone, R. Ecosystems: Have desert researchers discovered a hidden loop in the carbon cycle? Science 2008, 320, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, J.; Pang, Y.; Liu, J. Soil inorganic carbon sequestration following afforestation is probably induced by pedogenic carbonate formation in Northwest China. Front. Plant. Sci. 2017, 8, 1282. [Google Scholar] [CrossRef]
- Gaur, M.K.; Squires, V.R. (Eds.) Climate Variability Impacts on Land Use and Livelihoods in Drylands; Springer International Publishing: Cham, Germany, 2018; ISBN 9783319566801. [Google Scholar]
- Chai, Q.; Qin, A.; Gan, Y.; Yu, A. Higher yield and lower carbon emission by intercropping Maize with Rape, Pea, and Wheat in arid irrigation areas. Agron. Sustain. Dev. 2014, 34, 535–543. [Google Scholar] [CrossRef]
- Ewing, S.A.; Sutter, B.; Owen, J.; Nishiizumi, K.; Sharp, W.; Cliff, S.S.; Perry, K.; Dietrich, W.; McKay, C.P.; Amundson, R. A threshold in soil formation at Earth’s arid–hyperarid transition. Geochim. Cosmochim. Acta 2006, 70, 5293–5322. [Google Scholar] [CrossRef]
- Monger, H.C. Soils as generators and sinks of inorganic carbon in geologic time. In Soil Carbon; Hartemink, A.E., McSweeney, K., Eds.; Springer International Publishing: Cham, Germany, 2014; pp. 27–36. ISBN 9783319040837. [Google Scholar]
- Birkeland, P.W. Soils and Geomophology; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Nyachoti, S.; Jin, L.; Tweedie, C.E.; Ma, L. Insight into factors controlling formation rates of pedogenic carbonates: A combined geochemical and isotopic approach in dryland soils of the US Southwest. Chem. Geol. 2019, 527, 118503. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Belnap, J.; Marion, G. On carbon sequestration in desert ecosystems. Glob. Chang. Biol. 2009, 15, 1488–1490. [Google Scholar] [CrossRef]
- Arkley, R.J. Calculation of carbonate and water movement in soil from climatic data. Soil Sci. 1963, 96, 239–248. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Pilmanis, A.M. Plant-soil interactions in deserts. In Plant-Induced Soil Changes: Processes and Feedbacks; Springer: Dordrecht, The Netherlands, 1998; pp. 169–187. ISBN 9789048150847. [Google Scholar]
- Jenny, H. Calcium in the soil: III. Pedologic Relations. Soil Sci. Soc. Am. J. 1942, 6, 27–35. [Google Scholar] [CrossRef]
- Doner, H.E.; Lynn, W.C. Carbonate, halide, sulfate, and sulfide minerals. In SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1989; pp. 279–330. ISBN 9780891188605. [Google Scholar]
- Okazaki, M.; Setoguchi, H.; Aoki, H.; Suga, S. Application of soft X-Ray microradiography to observation of Cystoliths in the leaves of various higher plants. Bot. Mag. Tokyo 1986, 99, 281–287. [Google Scholar] [CrossRef]
- Wright, V.P. The role of fungal biomineralization in the formation of early carboniferous soil fabrics. Sedimentology 1986, 33, 831–838. [Google Scholar] [CrossRef]
- Monger, H.C.; Daugherty, L.A.; Lindemann, W.C.; Liddell, C.M. Microbial precipitation of pedogenic calcite. Geology 1991, 19, 997. [Google Scholar] [CrossRef]
- Liu, X.; Monger, H.C.; Whitford, W.G. Calcium carbonate in termite galleries–biomineralization or upward transport? Biogeochemistry 2007, 82, 241–250. [Google Scholar] [CrossRef]
- Chaparro-Acuña, S.P.; Becerra-Jiménez, M.L.; Martínez-Zambrano, J.J.; Rojas-Sarmiento, H.A. Soil bacteria that precipitate calcium carbonate: Mechanism and applications of the process. Acta Agron. 2018, 67, 277–288. [Google Scholar] [CrossRef]
- Lin, M.L.; Yen, T.B.; Huang, L.L. Formation of calcium carbonate deposition in the cotyledons during the germination of Justicia Procumbens L. (Acanthaceae) seeds. Taiwania 2004, 49, 250–262. [Google Scholar]
- Wang, H.T.; Xue, P.P.; He, X.D.; Gao, Y.B.; Li, Y.H.; Duan, X.C. Change of soil substrates in Artemisia ordosica succession series. Acta Sci. Nat. Univ. Nankaiensis 2007, 40, 87–91. [Google Scholar]
- Zhang, N.; He, X.D.; Gao, Y.B.; Li, Y.H.; Wang, H.T.; Ma, D.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere 2010, 20, 229–235. [Google Scholar] [CrossRef]
- Lobova, E. Soils of the Desert Zone of the USSR; Israel Program for Scientific Translation: Jerusalem, Israel, 1967. [Google Scholar]
- Phillips, S.E.; Milnes, A.R.; Foster, R.C. Calcified filaments: An example of biological influences in the formation of calcretes in South Australia. Aust. J. Soil Res. 1987, 25, 405–428. [Google Scholar] [CrossRef]
- Lal, R. Sequestering carbon in soils of arid ecosystems: Sequestering carbon in soils. Land Degrad. Dev. 2009, 20, 441–454. [Google Scholar] [CrossRef]
- Marion, G.M.; Schlesinger, W.H.; Fonteyn, P.J. Caldep: A regional model for soil CaCO3 (Caliche) deposition in Southwestern deserts. Soil Sci. 1985, 139, 468–481. [Google Scholar] [CrossRef]
- Sobecki, T.M.; Wilding, L.P. Formation of calcic and argillic horizons in selected soils of the Texas Coast Prairie. Soil Sci. Soc. Am. J. 1983, 47, 707–715. [Google Scholar] [CrossRef]
- Rabenhorst, M.C.; Wilding, L.P. Pedogenesis on the Edwards plateau, Texas: I. Nature and continuity of parent material. Soil Sci. Soc. Am. J. 1986, 50, 678–687. [Google Scholar] [CrossRef]
- Sanderman, J. Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agric. Ecosyst. Environ. 2012, 155, 70–77. [Google Scholar] [CrossRef]
- Lal, R.; Kimble, J.M.; Kimble, H. Pedogenic Carbonates and the Global Carbon Cycle; CRC Press (LewisPublishers): London, UK, 2000. [Google Scholar]
- Emmerich, W.E. Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric. For. Meteorol. 2003, 116, 91–102. [Google Scholar] [CrossRef]
- Entry, J.A.; Sojka, R.E.; Shewmarker, G.E. Irrigation increase inorganic carbon in agriculture soils. Environ. Manag. 2004, 33, 309–317. [Google Scholar]
- Sverdrup, H.; Warfvinge, P. Weathering of primary silicate minerals in the natural soil environment in relation to a chemical-weathering model. Water Air Soil Pollut. 1988, 38, 387–408. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Kelly, E.F.; Merritts, D.M.; Amundson, R.G. Carbondioxide consumption during soil development. Biogeochemistry 1994, 24, 115–127. [Google Scholar] [CrossRef]
- Khokhlova, O.S.; Arlashina, E.A.; Kovalevskaya, I.S. The effect of irrigation on the carbonate status of Chernozems of Central Precaucasus (Russia). Soil Technol. 1997, 11, 171–184. [Google Scholar] [CrossRef]
- Suarez, D.L. Impact of agriculture on CO2 as affected by changes in inorganic carbon. In Global Climate Change and Pedogenic Carbonates; Lal, R., Kimble, J.M., Eswaran, H., Stewart, B.A., Eds.; NHBS: Totnes, UK, 2000; pp. 257–272. [Google Scholar]
- Wohlfahrt, G.; Fenstermaker, L.F.; Arnone, J.A. Large annual net ecosystem CO2 uptake of a Mojave desert ecosystem. Global Change Biol. 2008, 14, 1475–1487. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Li, X.; Wang, J.; Xu, M.; Li, D. Dynamics of soil organic and inorganic carbon in the cropland of Upper Yellow River Delta, China. Sci. Rep. 2016, 6, 36105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jobbágy, E.G.; Richter, D.D.; Trumbore, S.E.; Jackson, R.B. Agricultural acceleration of soil carbonate weathering. Glob. Chang. Biol. 2020, 26, 5988–6002. [Google Scholar] [CrossRef] [PubMed]
- Raheb, A.; Heidari, A.; Mahmoodi, S. Organic and inorganic carbon storage in soils along an arid to dry sub-humid climosequence in Northwest of Iran. Catena 2017, 153, 66–74. [Google Scholar] [CrossRef]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic Carbonates: Forms and formation processes. Earth Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421. [Google Scholar] [CrossRef]
- Mi, N.A.; Wang, S.; Liu, J.; Yu, G.; Zhang, W.; Jobbágy, E. Soil inorganic carbon storage pattern in China. Glob. Chang. Biol. 2008, 14, 2380–2387. [Google Scholar] [CrossRef]
- Tan, W.F.; Zhang, R.; Cao, H.; Huang, C.Q.; Yang, Q.K.; Wang, M.K.; Koopal, L.K. Soil inorganic carbon stock under different soil types and land uses on the Loess Plateau region of China. Catena 2014, 121, 22–30. [Google Scholar] [CrossRef]
- Feng, Q.; Cheng, G.D.; Kunihiko, E. Carbon storage in desertified lands: A case study from North China. Geo J. 2000, 51, 181–189. [Google Scholar]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s “Grain-for-Green” program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef]
- Hombegowda, H.C.; van Straaten, O.; Köhler, M.; Hölscher, D. On the rebound: Soil organic carbon stocks can bounce back to near forest levels when agroforests replace agriculture in Southern India. Soil 2016, 2, 13–23. [Google Scholar] [CrossRef]
- Chang, R.; Fu, B.; Liu, G.; Wang, S.; Yao, X. The effects of afforestation on soil organic and inorganic carbon: A case study of the Loess Plateau of China. Catena 2012, 95, 145–152. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, Y.; Wang, Y.; Wei, X.; Wang, Y.; Cui, B.; Zhou, W. Natural vegetation restoration is more beneficial to soil surface organic and inorganic carbon sequestration than tree plantation on the Loess Plateau of China. Sci. Total Environ. 2014, 485–486, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhuang, Q.; Yu, D.; Shi, X.; Xing, S.; Xiong, D.; Liu, Y. Quantification of the soil organic carbon balance in the Tai-Lake paddy soils of China. Soil Tillage Res. 2016, 155, 95–106. [Google Scholar] [CrossRef]
- Rasmussen, C. Distribution of soil organic and inorganic carbon pools by biome and soil taxa in Arizona. Soil Sci. Soc. Am. J. 2006, 70, 256–265. [Google Scholar] [CrossRef]
- Gao, Y.; Dang, P.; Zhao, Q.; Liu, J.; Liu, J. Effects of vegetation rehabilitation on soil organic and inorganic carbon stocks in the Mu Us Desert, Northwest China. Land Degrad. Dev. 2018, 29, 1031–1040. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Murty, D.; Kirschbaum, M.U.F.; Mcmurtrie, R.E.; Mcgilvray, H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob. Chang. Biol. 2002, 8, 105–123. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Huang, C.; Wang, B.; Cao, H.; Koopal, L.K.; Tan, W. Effect of different vegetation cover on the vertical distribution of soil organic and inorganic carbon in the Zhifanggou watershed on the Loess Plateau. Catena 2016, 139, 191–198. [Google Scholar] [CrossRef]
- West, T.O.; McBride, A.C. The contribution of agricultural lime to carbondioxide emissions in the United States: Dissolution, transport, and net emissions. Agric. Ecosyst. Environ. 2005, 108, 145–154. [Google Scholar] [CrossRef]
- Woodbury, P.B.; Heath, L.S.; Smith, J.E. Effects of land use change on soil carbon cycling in the Conterminous United States from1900 to 2050. Glob. Biogeochem. Cycles 2007, 21, GB3006. [Google Scholar] [CrossRef]
- Monger, H.; Martinez-Rios, J. Inorganic Carbon Sequestration in Grazing Lands. In The Potential of U.S. Grazing Lands to Sequester Carbon and Mitigate the Greenhouse Effect; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Su, Y.Z.; Wang, X.F.; Yang, R.; Lee, J. Effects of sandy decertified land rehabilitation on soil carbon sequestration and aggregation in an arid region in China. J. Environ. 2010, 91, 2109–2116. [Google Scholar]
- Lu, T.; Wang, X.; Zhang, W. Total and dissolved soil organic and inorganic carbon and their relationships in typical Loess cropland of Fengu Basin. Geosci. Lett. 2020, 7, 17. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Post, C.J. Effects of land use on soil inorganic carbon stocks in the Russian Chernozem. J. Environ. Qual. 2006, 35, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonate formation: Recrystallization versus migration-process rates and periods assessed by 14C labeling. Glob. Biogeochem. Cycles 2012, 26, GB1018. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of Poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Wang, Z.P.; Han, X.G.; Chang, S.X.; Wang, B.; Yu, Q.; Hou, L.Y.; Li, L.H. Soil organic and inorganic carbon contents under various land uses across a transect of Continental Steppes in inner Mongolia. Catena 2013, 109, 110–117. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Xu, M.; Zhang, W.; Fan, T.; Zhang, J. Carbon accumulation in arid croplands of Northwest China: Pedogenic carbonate exceeding organic carbon. Sci. Rep. 2015, 5, 11439. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, M.G.; Wang, J.P.; Zhang, W.J.; Yang, X.Y.; Huang, S.M.; Liu, H. Fertilization enhancing carbon sequestration as carbonate in arid cropland: Assessments of long-term experiments in Northern China. Plant. Soil 2014, 380, 89–100. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Guo, T.; Zhang, P.; Wang, J. Soil organic and inorganic carbon in the Loess profiles of Lanzhou area: Implications of deep soils. Catena 2015, 126, 68–74. [Google Scholar] [CrossRef]
- Raymond, P.A.; Cole, J.J. Increase in the export of alkalinity from North America’s largest river. Science 2003, 301, 88–91. [Google Scholar] [CrossRef]
- Chen, J.; He, D.; Cui, S. The response of river water quality and quantity to the development of irrigated agriculture in the last 4 decades in the Yellow River Basin, China. Water Resour. Res. 2003, 39, 1047. [Google Scholar] [CrossRef]
- Denef, K.; Stewart, C.E.; Brenner, J.; Paustian, K. Does long-term center-pivot irrigation increase soil carbon stocks in semi-arid agro-ecosystems? Geoderma 2008, 145, 121–129. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Schlegel, A.J. Crop rotation effect on soil carbon and nitrogen stocks under limited irrigation. Agron. J. 2012, 104, 1265–1273. [Google Scholar] [CrossRef]
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Carbonate recrystallization in root-free soil and rhizosphere of Triticum Aestivum and Lolium Perenne estimated by 14C labeling. Biogeochemistry 2010, 103, 209–222. [Google Scholar] [CrossRef]
- Suarez, D.L. Ion activity products of calcium carbonate in waters below the root zone. Soil Sci. Soc. Am. J. 1977, 41, 310–315. [Google Scholar] [CrossRef]
- Suarez, D.L. Inorganic Carbon: Land Use Impacts. In Encyclopedia of Soil Science; Lal, R., Ed.; Taylor and Francis: Abingdon, UK, 2006; pp. 597–895. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Rengel, Z. (Ed.) Handbook of Soil Acidity; Marcel Dekker: New York, NY, USA, 2003; ISBN 9780824747398. [Google Scholar]
- Meng, H.Q.; Xu, M.G.; Lü, J.L.; He, X.H.; Li, J.W.; Shi, X.J.; Peng, C.; Wang, B.R.; Zhang, H.M. Soil pH dynamics and nitrogen transformations under long-term chemical fertilization in four Typical Chinese croplands. J. Integr. Agric. 2013, 12, 2092–2102. [Google Scholar] [CrossRef]
- Ramnarine, R.; Wagner-Riddle, C.; Dunfield, K.E.; Voroney, R.P. Contributions of carbonates to soil CO2 emissions. Can. J. Soil Sci. 2012, 92, 599–607. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Yang, C.; Wang, Y.; Xue, H.; Niu, Y. Rates of soil acidification in tea plantations and possible causes. Agric. Ecosyst. Environ. 2016, 233, 60–66. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, C.; Ma, W.; Wang, S.; Wang, S.; Han, W.; Mohammat, A.; Robinson, D.; Smith, P. Significant soil acidification across Northern China’s grasslands during 1980′s-2000′s. Glob. Chang. Biol. 2012, 18, 2292–2300. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2003; pp. 215–272. ISBN 9780120007967. [Google Scholar]
- Shi, Y.; Baumann, F.; Ma, Y.; Song, C.; Kühn, P.; Scholten, T.; He, J.S. Organic and inorganic carbon in the topsoil of the Mongolian and Tibetan grasslands: Pattern, control and implications. Biogeosciences 2012, 9, 2287–2299. [Google Scholar] [CrossRef]
- Adams, T.M.; Adams, S.N. The effects of liming and soil pH on carbon and nitrogen contained in the soil biomass. J. Agric. Sci. 1983, 101, 553–558. [Google Scholar] [CrossRef]
- Jin, S.; Tian, X.; Wang, H. Hierarchical responses of soil organic and inorganic carbon dynamics to soil acidification in a dryland agroecosystem, China. J. Arid Land 2018, 10, 726–736. [Google Scholar] [CrossRef]
- Riley, D.; Barber, S.A. Bicarbonate accumulation and pH changes at Soybean (Glycine max Merr) root-soil interface. Soil Sci. Soc. Am. Proc. 1969, 33, 905–908. [Google Scholar] [CrossRef]
- Mubarak, A.R.; Nortcliff, S. Calcium carbonate solubilization through H-proton release from some legumes grown in calcareous saline-sodic soils. Land Degrad. Dev. 2010, 21, 24–31. [Google Scholar] [CrossRef]
- Helyar, K.R.; Cregan, P.D.; Godyn, D.L. Soil acidity in New South Wale– Current pH values and estimates of acidification rates. Aust. J. Soil Res. 1990, 28, 523–537. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 Bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Buysse, P.; Roisin, C.; Aubinet, M. Fifty years of contrasted residue management of an agricultural crop: Impacts on the soil carbon budget and on soil heterotrophic respiration. Agric. Ecosyst. Environ. 2013, 167, 52–59. [Google Scholar] [CrossRef]
- Zhou, L.K. Soil Enzyme; Science Press: Beijing, China, 1987. [Google Scholar]
- Harrison, R.B.; Footen, P.W.; Strahm, B. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole ecosystem response to management and global change. Forest Sci. 2011, 57, 67–76. [Google Scholar]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423. [Google Scholar] [CrossRef]
- Xie, H.L.; Wang, Y.G.; Li, Y. Study on carbon leaching test of irrigation in arid area. Arid Zone 2015, 32, 903–909. [Google Scholar]
- McLauchlan, K. The nature and longevity of agricultural impacts on soil carbon and nutrients: A review. Ecosystems 2006, 9, 1364–1382. [Google Scholar] [CrossRef]
- Dessert, C.; Dupré, B.; Gaillardet, J.; François, L.M.; Allègre, C.J. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 2003, 202, 257–273. [Google Scholar] [CrossRef]
- Brock, A.L.; Buck, B.J. Polygenetic development of the Mormon Mesa, NV petrocalcic horizons: Geomorphic and paleoenvironmental interpretations. Catena 2009, 77, 65–75. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium mediated stabilization of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry. Geotimes 1997, 423, 44. [Google Scholar]
- Huang, B.; Wang, J.G.; Jing, H.Y.; Xu, S.W. Effects of long-term application of fertilizer on carbon storage in calcareous meadow soil. J. Agro-Environ. 2006, 25, 161–164. [Google Scholar]
- Zeng, J.; Guo, T.W.; Bao, G.X.; Wang, Z.; Sun, J.H. Effections of soil organic carbon and soil inorganic carbon under long-term fertilization. Soil Fertil. Sci. 2008, 2, 11–14. [Google Scholar]
- Kolosz, B.W.; Sohi, S.P.; Manning, D.A.C. CASPER: A modelling framework to link mineral carbonation with the turnover of organic matter in soil. Comput. Geosci. 2019, 124, 58–71. [Google Scholar] [CrossRef]
- Demoling, F.; Figueroa, D.; Bååth, E. Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 2007, 39, 2485–2495. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Lu, T.; Shi, H.; Zhao, Y. Influences of soil properties and hydrological processes on soil carbon dynamics in the cropland of North China Plain. Agric. Ecosyst. Environ. 2020, 295, 106886. [Google Scholar] [CrossRef]
- Virto, I.; Gartzia-Bengoetxea, N.; Fernández-Ugalde, O. Role of organic matter and carbonates in soil aggregation estimated using laser diffractometry. Pedosphere 2011, 21, 566–572. [Google Scholar] [CrossRef]
- Shi, H.J.; Wang, X.J.; Zhao, Y.J.; Xu, M.G.; Li, D.W.; Guo, Y. Relationship between soil inorganic carbon and organic carbon in the Wheat-Maize cropland of the North China Plain. Plant. Soil 2017, 418, 423–436. [Google Scholar] [CrossRef]
- Evans, R.D.; Koyama, A.; Sonderegger, D.L.; Charlet, T.N.; Newingham, B.A.; Fenstermaker, L.F.; Harlow, B.; Jin, V.L.; Ogle, K.; Smith, S.D.; et al. Greater ecosystem carbon in the Mojave Desert after ten years exposure to elevated CO2. Nat. Clim. Chang. 2014, 4, 394–397. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Tang, L.S.; Lan, Z.D.; Li, Y. A downward CO2 flux seems to have nowhere to go. Biogeosciences 2014, 11, 6251–6262. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.G.; Houghton, R.A.; Tang, L.S. Hidden carbon sink beneath desert. Geophys. Res. 2015, 42, 5880–5887. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Kessler, T.J.; Harvey, C.F. The global flux of carbondioxide into groundwater. Geophys. Res. Lett. 2001, 28, 279–282. [Google Scholar] [CrossRef]
- Amiaz, Y.; Sorek, S.; Enzel, Y.; Dahan, O. Solute transport in the vadose zone and groundwater during flash floods: Floods impact on subsurface solute transport. Water Resour. Res. 2011, 47, W10513. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley: New York, NY, USA, 1979. [Google Scholar]
- Scanlon, B.R.; Keese, K.E.; Flint, A.L.; Flint, L.E.; Gaye, C.B.; Edmunds, W.M.; Simmers, I. Global synthesis of groundwater recharge in semiarid and arid regions. Hydrol. Process. 2006, 20, 3335–3370. [Google Scholar] [CrossRef]
- Karberg, N.J.; Pregitzer, K.S.; King, J.S.; Friend, A.L.; Wood, J.R. Soil carbon dioxide partial pressure and dissolved inorganic carbonate chemistry under elevated carbon dioxide and ozone. Oecologia 2005, 142, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.D.; Markewitz, D. How deep is soil? BioScience 1995, 45, 600–609. [Google Scholar] [CrossRef]
- Mielnick, P.; Dugas, W.A.; Mitchell, K.; Havstad, K. Long-Term Measurements of CO2 Flux and Evapotranspiration in a Chihuahuan Desert Grassland. J. Arid Environ. 2005, 60, 423–436. [Google Scholar] [CrossRef]
- Inglima, I.; Alberti, G.; Bertolini, T.; Vaccari, F.P.; Gioli, B.; Miglietta, F.; Cotrufo, M.F.; Peressotti, A. Precipitation Pulses Enhance Respiration of Mediterranean Ecosystems: The Balance between Organic and Inorganic Components of Increased Soil CO2 efflux. Glob. Chang. Biol. 2009, 15, 1289–1301. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Zhai, C.; Li, C.Z.L. CO2 Absorption by Alkaline Soils and Its Implication to the Global Carbon Cycle. Environ. Geol. 2008, 56, 953–961. [Google Scholar] [CrossRef]
- Hamerlynck, E.P.; Scott, R.L.; Sánchez-Cañete, E.P.; Barron-Gafford, G.A. Nocturnal Soil CO2 Uptake and Its Relationship to Subsurface Soil and Ecosystem Carbon Fluxes in a Chihuahuan Desert Shrubland: Nocturnal Desert Soil CO2UPTAKE. J. Geophys. Res. Biogeosci. 2013, 118, 1593–1603. [Google Scholar] [CrossRef]
- Ball, B.A.; Virginia, R.A.; Barrett, J.E.; Parsons, A.N.; Wall, D.H. Interactions between Physical and Biotic Factors Influence CO2 Flux in Antarctic Dry Valley Soils. Soil Biol. Biochem. 2009, 41, 1510–1517. [Google Scholar] [CrossRef]
- Roland, M.; Serrano-Ortiz, P.; Kowalski, A.S.; Goddéris, Y.; Sánchez-Cañete, E.P.; Ciais, P.; Domingo, F.; Cuezva, S.; Sanchez-Moral, S.; Longdoz, B.; et al. Atmospheric Turbulence Triggers Pronounced Diel Pattern in Karst Carbonate Geochemistry. Biogeosciences 2013, 10, 5009–5017. [Google Scholar] [CrossRef]
- Breecker, D.O.; Sharp, Z.D.; McFadden, L.D. Seasonal Bias in the Formation and Stable Isotopic Composition of Pedogenic Carbonate in Modern Soils from Central New Mexico, USA. Geol. Soc. Am. Bull. 2009, 121, 630–640. [Google Scholar] [CrossRef]
- Roby, M.C.; Scott, R.L.; Barron-Gafford, G.A.; Hamerlynck, E.P.; Moore, D. Environmental and Vegetative Controls on Soil CO2 -Efflux in Three Semi-Arid Ecosystems. Soil Syst. 2019, 3, 6. [Google Scholar] [CrossRef]
- Soper, F.M.; McCalley, C.K.; Sparks, K.; Sparks, J.P. Soil Carbon Dioxide Emissions from the Mojave Desert: Isotopic Evidence for a Carbonate Source. Geophys. Res. Lett. 2017, 44, 245–251. [Google Scholar] [CrossRef]
- Kowalski, A.S.; Serrano-Ortiz, P.; Janssens, I.A.; Sanchez-Moral, S.; Cuezva, S.; Domingo, F.; Were, A. Can Flux Tower Research Neglect Geochemical CO2 Exchange. Aldos-Arboledas A 2008, 148, 1045–1054. [Google Scholar] [CrossRef]
- Plestenjak, G.; Eler, K.; Vodnik, D.; Ferlan, M.; Čater, M.; Kanduč, T.; Simončič, P.; Ogrinc, N. Sources of Soil CO2 in Calcareous Grassland with Woody Plant Encroachment. J. Soils Sediments 2012, 12, 1327–1338. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naorem, A.; Jayaraman, S.; Dalal, R.C.; Patra, A.; Rao, C.S.; Lal, R. Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils—A Review. Agriculture 2022, 12, 1256. https://doi.org/10.3390/agriculture12081256
Naorem A, Jayaraman S, Dalal RC, Patra A, Rao CS, Lal R. Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils—A Review. Agriculture. 2022; 12(8):1256. https://doi.org/10.3390/agriculture12081256
Chicago/Turabian StyleNaorem, Anandkumar, Somasundaram Jayaraman, Ram C. Dalal, Ashok Patra, Cherukumalli Srinivasa Rao, and Rattan Lal. 2022. "Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils—A Review" Agriculture 12, no. 8: 1256. https://doi.org/10.3390/agriculture12081256