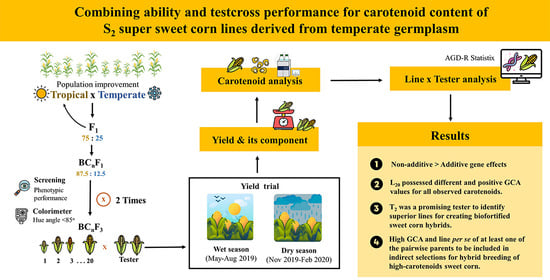
Combining Ability and Testcross Performance for Carotenoid Content of S2 Super Sweet Corn Lines Derived from Temperate Germplasm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Field Experiment
2.3. Soil and Weather Data
2.4. Carotenoid Extraction and Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Line and Tester Analysis
3.2. Evaluation of Hybrid Performance
3.3. General Combining Ability (GCA) and Specific Combining Ability (SCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruanjaichon, V.; Khammona, K.; Thunnom, B.; Suriharn, K.; Kerdsri, C.; Aesomnuk, W.; Yongsuwan, A.; Chaomueang, N.; Thammapichai, P.; Arikit, S.; et al. Identification of gene associated with sweetness in corn (Zea mays L.) by genome-wide association study (GWAS) and development of a functional SNP marker for predicting sweet corn. Plants 2021, 10, 1239. [Google Scholar] [CrossRef]
- Mehta, B.K.; Muthusamy, V.; Baveja, A.; Chauhan, H.S.; Chhabra, R.; Bhatt, V.; Chand, G.; Zunjare, R.U.; Singh, A.K.; Hossain, F. Composition analysis of lysine, tryptophan and provitamin-A during different stages of kernel development in biofortified sweet corn. J. Food Comp. Anal. 2020, 94, 103625. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 30 June 2020).
- Swapna, G.; Jadesha, G.; Mahadevu, P. Sweet corn-A future healthy human nutrition food. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 3859–3865. [Google Scholar] [CrossRef]
- Sheng, S.; Li, T.; Liu, R.H. Corn phytochemicals and their health benefits. J. Food Sci. 2018, 7, 185–195. [Google Scholar]
- Palozza, P.; Krinsky, N.I. Antioxidant effects of carotenoids in vivo and in vitro: An overview. Meth. Enzymol. 1992, 213, 403–452. [Google Scholar]
- Moeller, S.M.; Jacques, P.F.; Blumberg, J.B. The potential role of dietary xanthophylls in cataract and age-related macular degeneration. J. Am. Coll. Nutr. 2000, 19, 522S–527S. [Google Scholar] [CrossRef]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, A.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated carotenoids lutein and progression of early atherosclerosis. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef]
- Trono, D. Carotenoids in cereal food crops: Composition and retention throughout grain storage and food processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary source of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Baseggio, M.; Murray, M.; Magallanes-Lundback, M.; Nicholas Kaczmar, N.; Chamness, J.; Buckler, E.S.; Smith, M.E.; Penna, D.D.; Tracy, W.F.; Gore, M.A. Natural variation for carotenoids in fresh kernels is controlled by uncommon variants in sweet corn. Plant Genome 2020, 13, e20008. [Google Scholar] [CrossRef]
- Fanning, K.; Martin, I.; Wong, L.; Keating, V.; Pun, S.; Hare, O.J. Screening sweetcorn for enhanced zeaxanthin concentration. J. Sci. Food Agric. 2010, 90, 91–96. [Google Scholar] [CrossRef]
- Li, R.; Xiao, L.H.; Wang, J.; Lu, Y.L.; Rong, T.Z.; Pan, G.T.; Wu, Y.Q.; Tang, Q.; Lan, H.; Cao, M.J. Combining ability and parent-offspring correlation of maize (Zea may L.) grain β-carotene content with a complete diallel. J. Integr. Agric. 2013, 12, 19–26. [Google Scholar] [CrossRef]
- Khokhar, E.S.; Shakeel, A.; Maqbool, M.A.; Abuzar, M.K.; Zareen, S.; Aamir, S.A.; Asadullah, M. Studying combining ability and heterosis in different cotton (Gossypium hirsutum L.) genotypes for yield and yield contributing traits. Pak. J. Agric. Sci. 2018, 31, 55–68. [Google Scholar] [CrossRef]
- Rojas, B.A.; Sprague, G.F. A comparison of variance components in corn yield trials. III general and specific combining ability and their interactions with locations and years. Agron. J. 1952, 44, 462–466. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics, 3rd ed.; Longman Scientific and Technical, Co.: New York, NY, USA, 1989. [Google Scholar]
- Sharma, H.C.; Dhillan, M.K.; Reddy, B.V.S. Expression of resistance to Atherigona soccata in F1 hybrids involving shoot fly-resistant and susceptible cytoplasmic male-sterile and restorer lines of sorghum. Plant Breed. 2006, 125, 473–477. [Google Scholar] [CrossRef]
- Elmyhum, M. Estimation of combining ability and heterosis of quality protein maize inbred lines. Afr. J. Agric. Res. 2013, 8, 6309–6317. [Google Scholar]
- Amin, M.N.; Amirurzaman, M.; Ahmed, A.; Ali, M.R. Evaluation of inbred lines of maize (Zea mays L.) through line x tester method. Bangladesh J. Agric. Res. 2014, 39, 675–683. [Google Scholar] [CrossRef]
- Kahriman, F.; Egesel, C.Ö.; Orhun, G.E.; Alaca, B.; Avci, F. Comparison of graphical analyses for maize genetic experiments: Application of biplots and polar plot to line x tester design. Chil. J. Agric. Res. 2016, 76, 285–293. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Aslam, M.; Khan, M.S.; Beshir, A.; Ahan, M. Evaluation of single cross yellow maize hybrids for agronomic and carotenoid traits. Int. J. Agric. Biol. 2017, 19, 1087–1098. [Google Scholar] [CrossRef]
- Fountain, J.C.; Abbas, H.K.; Scully, B.T.; Li, H.; Lee, R.D.; Kemerait, R.C.; Guo, B. Evaluation of maize inbred lines and topcross progeny for resistance to pre-harvest aflatoxin contamination. Crop J. 2019, 7, 118–125. [Google Scholar] [CrossRef]
- Zebire, D.; Menkir, A.; Adetimirin, V.; Mengesha, W.; Meseka, S.; Gedil, M. Effectiveness of yellow maize testers with varying resistance reactions to Striga hermonthica for evaluating the combining ability of maize inbred lines. Agronomy 2020, 10, 1276. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Aslam, M.; Beshir, A.; Khan, M.S. Breeding for provitamin A biofortification of maize (Zea mays L.). Plant Breed. 2018, 137, 451–469. [Google Scholar] [CrossRef]
- Egesel, C.O.; Wong, J.C.; Lambert, R.J.; Rocheford, T.R. Combining ability of maize inbred for carotenoid and tocopherols. Crop Sci. 2003, 43, 818–823. [Google Scholar] [CrossRef]
- Harjes, C.E.; Rocheford, T.R.; Bai, L.; Brutnell, T.P.; Kandianis, C.B.; Sowinski, S.G.; Stapleton, A.E.; Vallabhaneni, R.; Williams, M.; Wurtzel, E.T.; et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 2008, 319, 330–333. [Google Scholar] [CrossRef]
- da Silva Messias, R.; Galli, V.; Silva, S.D.D.A.; Rombaldi, C.V. Carotenoid biosynthetic and catabolic pathways: Gene expression and carotenoid content in grains of maize landraces. Nutrients 2014, 6, 546–563. [Google Scholar] [CrossRef]
- Halilu, A.D.; Ado, S.G.; Aba, D.A.; Usman, I.S. Genetics of carotenoids for provitamin A biofortification in tropical-adapted maize. Crop J. 2016, 4, 313–322. [Google Scholar] [CrossRef]
- Menkir, A.; Olowolafe, M.O.; Ingelbrecht, I.; Fawole, I.; BaduApraku, B.; Vroh, B.I. Assessment of testcross performance and genetic diversity of yellow endosperm maize lines derived from adapted × exotic backcrosses. Theor. Appl. Genet. 2006, 113, 90–99. [Google Scholar] [CrossRef]
- Maize Quick Carotenoid Extraction Protocol. Available online: http://www.cropsci.uiuc.edu/faculty/rocheford/quick_carotenoid_analysis_protocol.pdf (accessed on 20 June 2019).
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5–16. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; An International Rice Research Institute Book, Co., Inc.: New York, NY, USA, 1984. [Google Scholar]
- Statistix 10 Data analysis Software for Researchers. Available online: https://www.statistix.com (accessed on 11 November 2014).
- Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani Publishers: New Delhi, India, 1985. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Miranda, J.B. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010. [Google Scholar]
- Rodríguez, F.; Alvarado, G.; Pacheco, A.; Crossa, J.; Burgueno, J. AGD-R (Analysis of Genetic Designs with R for Windows) Version 5.0; International Maize and Wheat Improvement Center: Mexico City, Mexico, 2018. [Google Scholar]
- Mengesha, T.; Cao, F.B.; Wasim, I.; Wu, F.B. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing salt tolerance. Physiol. Plant 2019, 165, 134–143. [Google Scholar]
- Chander, S.; Guo, Y.; Zhang, Y.; Li, J. Comparison of nutritional traits variability in selected eighty-seven inbreds from Chinese maize (Zea mays L.) germplasm. J. Agric. Food Chem. 2008, 56, 6506–6511. [Google Scholar] [CrossRef]
- Muthusamy, V.; Hossain, F.; Thirunavukkarasu, N.; Saha, S.; Agrawal, P.K.; Gupta, H.S. Genetic analyses of kernel carotenoids in novel maize genotypes possessing rare allele of ß-carotene hydroxylase gene. Cereal Res. Commun. 2016, 44, 669–680. [Google Scholar] [CrossRef]
- Durães, N.N.L.; Crevelari, J.A.; Vettorazzi, J.C.F.; Ferreira, J.A.; de Abreu Santana, F.; Pereira, M.G. Combining ability for traits associated with yield and quality in super sweet corn (Zea mays L. saccharata). Crop Sci. 2017, 11, 1188–1194. [Google Scholar] [CrossRef]
- Suwarno, W.B.; Pixley, K.V.; Palacios-Rojas, N.; Kaeppler, S.M.; Babu, R. Formation of heterotic groups and understanding genetic effects in a provitamin A biofortified maize breeding program. Crop Sci. 2014, 54, 14–24. [Google Scholar] [CrossRef]
- Wong, J.C.; Lambert, R.J.; Wurtzel, E.T.; Rocheford, T.R. QTL and candidate genes phytoene synthase and zeta-carotene desaturase associated with the accumulation of carotenoids in maize. Theor. Appl. Genet. 2004, 108, 349–359. [Google Scholar] [CrossRef]
- Yan, J.; Kandianis, C.B.; Harjes, C.E.; Bai, L.; Kim, E.H.; Yang, X.; Skinner, D.; Fu, Z.; Mitchell, S.; Li, Q.; et al. Rare genetic variation at Zea mays crtRB1 increases β-carotene in maize grain. Nat. Genet. 2010, 42, 322–327. [Google Scholar] [CrossRef]
- Dhliwayo, T.; Palacios-Rojas, N.; Crossa, J.; Pixley, K.V. Effects of S1 recurrent selection for provitamin A carotenoid content for three open-pollinated maize cultivars. Crop Sci. 2014, 54, 2449–2460. [Google Scholar] [CrossRef]
- Khamkhoh, W.; Ketthaisong, D.; Lomthaisong, K.; Lertrat, K.; Suriharn, K. Recurrent selection method for improvement of lutein and zeaxanthin in orange waxy corn populations. Aust. J. Crop Sci. 2019, 13, 566–573. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Fanning, K.; Martin, I. Zeaxanthin biofortification of sweet-corn and factors affecting zeaxanthin accumulation and colour change. Arch. Biochem. Biophys. 2015, 572, 184–187. [Google Scholar] [CrossRef]
- Castellanos, J.S.; Hallauer, A.R.; Córdova, H.S. Relative performance of testers to identify elite lines of corn (Zea mays L.). Maydica 1998, 43, 217–226. [Google Scholar]
- Bernardo, R. Breeding for Quantitative Traits in Plants, 3rd ed.; Stemma Press: Woodbury, MN, USA, 2020. [Google Scholar]
- Fasahat, P.; Rajabi, A.; Rad, J.M.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 1–22. [Google Scholar] [CrossRef]
- Pswarayi, A.; Vivek, B.S. Combining ability amongst CIMMYT’s early maturing maize (Zea mays L.) germplasm under stress and non-stress conditions and identification of testers. Euphytica 2008, 162, 353–362. [Google Scholar] [CrossRef]
- Sprague, G.F.; Tatum, L.A. General vs. specific combining ability in single crosses of corn. J. Am. Soc. Agron. 1942, 34, 923–932. [Google Scholar] [CrossRef]

| No. | Code | Pedigree | Population | Description | Relative Carotenoid Content 1/ |
|---|---|---|---|---|---|
| 1 | L1 | Sugar-75/TSC-BC2-4-5 | 1 | Line | high |
| 2 | L2 | Sugar-75/TSC-BC2-6-2 | 1 | Line | low |
| 3 | L3 | Sugar-75/TSC-BC2-8-1 | 1 | Line | low |
| 4 | L4 | Sugar-75/TSC-BC2-10-5 | 1 | Line | high |
| 5 | L5 | Sugar-75/TSC-BC2-11-2 | 1 | Line | medium |
| 6 | L6 | Sugar-75/TSC-BC2-16-5 | 1 | Line | low |
| 7 | L7 | Sugar-75/TSC-BC2-22-1 | 1 | Line | medium |
| 8 | L8 | Sugar-75/TSC-BC2-25-1 | 1 | Line | medium |
| 9 | L9 | Sugar-75/TSC-BC2-28-3 | 1 | Line | high |
| 10 | L10 | Sugar-75/TSC-BC2-29-7 | 1 | Line | medium |
| 11 | L11 | Sugar-75/TSC-BC2-31-3 | 1 | Line | medium |
| 12 | L12 | Sugar-75/TSC-BC2-32-4 | 1 | Line | medium |
| 13 | L13 | Hibrix-3//KV/Delectable-BC1-11-9(2) | 2 | Line | medium |
| 14 | L14 | Hibrix-3//P4546/Montauk-BC1-10-6(1) | 2 | Line | medium |
| 15 | L15 | Hibrix-3//P4546/Montauk-BC1-11-7 | 2 | Line | high |
| 16 | L16 | Hibrix-3//P4546/Montauk-BC1-16-7 | 2 | Line | medium |
| 17 | L17 | Hibrix-3//Jumbo Sweet/Delectable-BC1-5-3 | 2 | Line | high |
| 18 | L18 | Hibrix-3//Jumbo Sweet/Delectable-BC1-5-5 | 2 | Line | medium |
| 19 | L19 | Hibrix-3//Jumbo Sweet/Delectable-BC1-13-5 | 2 | Line | medium |
| 20 | L20 | Hibrix-3//Jumbo Sweet/Delectable-BC1-17-4 | 2 | Line | high |
| 21 | T1 | Dr.Pek’s Wan 54 | Tester 1 | high | |
| 22 | T2 | Hibrix-53 | Tester 2 | low | |
| 23 | C1 | Dr.Pek’s 1351 | Check 1 | high | |
| 24 | C2 | Hibrix-59 | Check 2 | low |
| SOV | df 1/ | Mean Squares | ||||
|---|---|---|---|---|---|---|
| LUT 2/ | ZEA | β-CX | β-CT | TCC | ||
| Season (S) | 1 | 537.00 ** | 1566.00 ** | 39.50 ** | 5.77 ** | 1090.00 ** |
| Rep/S | 2 | 1.37 | 0.80 | 0.09 | 0.02 | 1.77 |
| Hybrid (H) | 39 | 110.00 ** | 178.00 ** | 24.61 ** | 0.83 ** | 316.00 ** |
| GCAL | 19 | 1042.00 ** | 213.00 ** | 30.24 ** | 0.91 ** | 264.00 ** |
| GCAT | 1 | 84.78 ** | 109.00 ** | 181.63 ** | 0.77 ** | 3383.00 ** |
| SCA | 19 | 87.61 ** | 149.00 ** | 10.71 ** | 0.76 ** | 207.00 ** |
| H × S | 39 | 31.11 ** | 82.19 ** | 7.47 ** | 0.49 ** | 149.90 ** |
| GCAL × S | 19 | 35.28 ** | 83.79 ** | 7.39 ** | 0.56 ** | 80.81 ** |
| GCAT × S | 1 | 84.78 ** | 0.00 | 0.19 | 0.06 | 7.71 |
| SCA × S | 19 | 24.18 ** | 85.48 ** | 7.94 ** | 0.43 ** | 226.00 ** |
| Pooled error | 156 | 0.81 | 0.77 | 0.19 | 0.02 | 2.88 |
| Proportion of genetic variance (%) | ||||||
| Ơ2A | 6.27 | 0 | 4.54 | 0 | 30.05 | |
| Ơ2D | 93.73 | 100 | 95.46 | 100 | 69.95 | |
| h2ns (%) | 6 | 0 | 4 | 0 | 24 | |
| Hybrids | Carotenoid Contents (µg/g of Dry Weight) | ||||
|---|---|---|---|---|---|
| LUT 1/ | ZEA | β-CX | β-CT | TCC | |
| T2 × L20 | 35.83 ab 2/ | 47.59 a | 12.85 a | 3.58 c | 74.01 a |
| T2 × L16 | 27.72 def | 33.04 gh | 7.81 g | 3.25 ef | 67.74 b |
| T2 × L14 | 36.01 ab | 29.79 klm | 7.96 g | 2.58 opq | 65.03 c |
| T2 × L11 | 24.64 hi | 30.35 kl | 9.37 de | 3.34 de | 64.08 cd |
| T1 × L20 | 28.10 de | 45.93 b | 10.66 c | 3.14 efg | 62.46 de |
| T2 × L5 | 34.90 b | 34.62 f | 9.02 ef | 3.12 fgh | 61.11 e |
| T2 × L17 | 36.03 ab | 27.77 op | 5.79 mno | 3.03 g–k | 61.08 e |
| T2 × L1 | 29.95 c | 32.54 hi | 6.73 h–l | 2.83 lmn | 60.61 e |
| T1 × L13 | 22.39 k–n | 35.86 e | 8.65 f | 2.62 opq | 60.20 e |
| T2 × L8 | 29.58 c | 29.08 mn | 6.67 h–l | 2.74 mno | 57.76 f |
| Mean of top ten hybrids | 34.56 | 43.21 | 11.89 | 3.36 | 60.45 |
| Check 1 | 23.30 jk | 20.96 u | 3.93 tu | 2.50 pqr | 42.94 o |
| Check 2 | 21.27 opq | 32.19 hij | 6.17 lmn | 2.84 j–n | 36.67 q |
| Mean of checks | 22.29 | 25.58 | 4.78 | 2.67 | 39.81 |
| Grand mean | 25.68 | 30.23 | 6.96 | 2.96 | 51.72 |
| C.V. (%) | 3.43 | 2.88 | 6.19 | 4.81 | 3.24 |
| Lines/ Testers | LUT 1/ | ZEA | β-CX | β-CT | TCC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean 2/ | GCA | Mean | GCA | Mean | GCA | Mean | GCA | Mean | GCA | |
| Lines | ||||||||||
| L1 | 17.36 c–g 3/ | 1.76 ** | 62.36 a | 1.89 ** | 4.34 d–h | −1.53 ** | 3.74 bcd | 0.89 ** | 71.78 bc | 8.71 ** |
| L2 | 12.97 e–h | 2.98 ** | 17.09 f–i | 3.89 ** | 5.69 c–h | 2.73 ** | 3.53 cde | −0.15 * | 29.34 m | 0.49 |
| L3 | 7.60 h | 1.04 * | 13.24 hi | −3.12 ** | 2.28 h | −1.26 ** | 2.54 efg | −0.49 ** | 22.03 n | −11.30 ** |
| L4 | 54.28 a | −1.25 ** | 55.54 a | −2.61 ** | 7.60 b–e | −1.65 ** | 3.04 d–g | −0.24 ** | 68.84 c | −9.69 ** |
| L5 | 14.36 d–h | 2.38 ** | 17.73 f–i | 2.29 ** | 9.74 ab | −0.00 | 4.30 abc | −0.21 ** | 40.71 hi | −0.98 |
| L6 | 13.44 e–h | −4.86 ** | 22.59 d–i | −3.69 ** | 2.34 gh | −0.77 ** | 3.10 d–g | −0.29 ** | 25.17 n | 5.40 ** |
| L7 | 18.51 c–f | −0.30 | 34.70 bc | −1.98 ** | 4.03 e–h | −1.46 ** | 2.67 efg | 0.10 | 39.28 ij | 5.57 ** |
| L8 | 9.34 fgh | 3.17 ** | 26.92 c–g | 2.38 ** | 3.65 fgh | −0.90 ** | 2.45 g | −0.63 ** | 36.50 jk | −7.03 ** |
| L9 | 22.26 b–e | −0.23 | 61.94 a | −3.21 ** | 9.99 ab | 0.43 * | 2.98 d–g | 1.38 ** | 82.24 a | 3.68 ** |
| L10 | 20.27 b–e | 0.75 * | 24.96 c–h | 2.39 ** | 13.25 a | 2.06 ** | 4.91 a | 0.79 ** | 43.15 gh | −2.81 ** |
| L11 | 20.78 b–e | −4.43 ** | 25.08 c–h | −3.36 ** | 5.74 c–h | −1.32 ** | 4.52 ab | 0.15 * | 48.59 f | −0.36 |
| L12 | 20.42 b–e | −2.82 ** | 34.10 bcd | −2.24 ** | 7.24 b–f | 0.75 ** | 4.73 a | −0.33 ** | 56.58 e | 2.38 ** |
| L13 | 9.21 fgh | 1.95 ** | 12.12 i | 2.47 ** | 4.09 e–h | 1.85 ** | 3.27 d–g | −0.40 ** | 33.89 kl | −1.56 * |
| L14 | 13.40 e–h | 2.32 ** | 19.78 f–i | −0.33 | 5.37 c–h | −0.41 * | 3.49 c–f | −0.51 ** | 36.59 jk | −1.53 * |
| L15 | 16.03 c–h | −5.78 ** | 19.63 f–i | −6.40 ** | 8.56 bc | −0.11 * | 4.68 ab | −0.15 * | 61.90 d | −2.83 ** |
| L16 | 16.82 c–h | −4.14 ** | 33.75 bcd | 3.20 ** | 2.36 gh | 0.70 ** | 2.70 efg | −0.21 ** | 44.86 fg | 10.34 ** |
| L17 | 44.59 a | 1.35 ** | 41.30 b | −4.52 ** | 7.99 bcd | −2.35 ** | 3.11 d–g | −0.51 ** | 72.54 bc | −1.68 * |
| L18 | 29.24 b | 2.24 ** | 28.80 c–f | −2.76 ** | 5.40 c–h | 0.12 | 2.94 d–g | −0.43 ** | 59.40 de | −0.43 |
| L19 | 8.51 gh | 2.28 ** | 15.91 ghi | 3.62 ** | 4.25 d–h | −0.89 ** | 4.59 ab | 0.52 ** | 32.62 lm | −12.55 ** |
| L20 | 24.27 bc | 1.59 ** | 31.92 b–e | 12.09 ** | 9.84 ab | 4.04 ** | 4.65 ab | 0.44 ** | 72.99 b | 16.16 ** |
| Testers | ||||||||||
| T1 | 23.30 bcd | −1.83 ** | 20.96 e–i | 0.86 ** | 3.93 eh | −1.10 ** | 2.51 fg | −0.09 ** | 42.94 ghi | −4.82 ** |
| T2 | 21.22 b–e | 1.83 ** | 32.19 b–e | −0.86 ** | 6.17 b–e | 1.10 ** | 2.84 d–g | 0.09 ** | 36.67 jk | 4.82 ** |
| SE Line | 0.58 | 0.61 | 0.28 | 0.10 | 1.20 | |||||
| SE Tester | 0.18 | 0.19 | 0.09 | 0.33 | 0.38 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasuwatthanakool, W.; Harakotr, B.; Jirakiattikul, Y.; Lomthaisong, K.; Suriharn, K. Combining Ability and Testcross Performance for Carotenoid Content of S2 Super Sweet Corn Lines Derived from Temperate Germplasm. Agriculture 2022, 12, 1561. https://doi.org/10.3390/agriculture12101561
Wasuwatthanakool W, Harakotr B, Jirakiattikul Y, Lomthaisong K, Suriharn K. Combining Ability and Testcross Performance for Carotenoid Content of S2 Super Sweet Corn Lines Derived from Temperate Germplasm. Agriculture. 2022; 12(10):1561. https://doi.org/10.3390/agriculture12101561
Chicago/Turabian StyleWasuwatthanakool, Worawan, Bhornchai Harakotr, Yaowapha Jirakiattikul, Khomsorn Lomthaisong, and Khundej Suriharn. 2022. "Combining Ability and Testcross Performance for Carotenoid Content of S2 Super Sweet Corn Lines Derived from Temperate Germplasm" Agriculture 12, no. 10: 1561. https://doi.org/10.3390/agriculture12101561