Understanding the Opportunities to Mitigate Carryover of Imidazolinone Herbicides in Lowland Rice
Abstract
:1. Introduction
2. Materials and Methods
3. Results
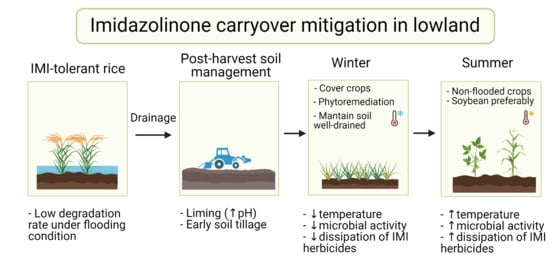
4. Management of Imidazolinone Residues in Lowlands
5. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.L. Herbicide Handbook, 10th ed.; Allen Press, Incorporated: Lawrence, KS, USA, 2014; p. 513. [Google Scholar]
- Shaner, D.L.; O’Connor, S.L. The Imidazolinone Herbicides; CRC Press: Madison, WI, USA, 1991. [Google Scholar]
- Cobucci, T.; Portela, C.M.O.; Silva, W.; Neto Monteiro, A. Comportamento de herbicidas com efeito residual em diferentes coberturas na cultura do feijoeiro. Planta Daninha 2004, 22, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Cobucci, T.; Portela, C.M.O.; Silva, W.; Neto Monteiro, A. Efeito residual de herbicidas em pré-plantio do feijoeiro, em dois sistemas de aplicação em plantio direto e sua viabilidade econômica. Planta Daninha 2004, 22, 583–590. [Google Scholar] [CrossRef]
- Vidal, R.A.; Nunes, A.L. Persistence of imazaquin associated to glyphosate or paraquat under no-tillage. Planta Daninha 2010, 28, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Sudianto, E.; Beng-Kah, S.; Ting-Xiang, N.; Saldain, N.E.; Scott, R.C.; Burgos, N.R. Clearfield® rice: Its development, success, and key challenges on a global perspective. Crop Prot. 2013, 49, 40–51. [Google Scholar] [CrossRef]
- Villa, S.C.C.; Marchezan, E.; Massoni, P.F.S.; Santos, F.M.; Avila, L.A.; Machado, S.L.O.; Telo, G.M. Controle de arroz-vermelho em dois genótipos de arroz (Oryza sativa) tolerantes a herbicidas do grupo das imidazolinonas. Planta Daninha 2006, 24, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.J.O.; Noldin, J.A.; Pinho, C.F.; Rossi, F.; Galon, L.; Almeida, G.F. Field persistence of (imazethapyr plus imazapic) to grain sorghum (Sorghum bicolor) planted in rotation after irrigated rice. Planta Daninha 2009, 27, 1015–1024. [Google Scholar] [CrossRef]
- Pinto, J.J.O.; Noldin, J.A.; Rosenthal, M.D.; Pinho, C.F.; Rossi, F.; Machado, A.; Piveta, L.; Galon, L. Residual activity of (imazethapyr plus imazapic) on ryegrass (Lolium multiflorum), following Clearfield (R) rice. Planta Daninha 2009, 27, 609–619. [Google Scholar] [CrossRef]
- Pinto, J.J.O.; Noldin, J.A.; Sousa, C.P.; Agostinetto, D.; Piveta, L.; Donida, A. Residual soil activity of imazethapyr plus imazapic to rice planted in rotation after clearfield rice (R). Planta Daninha 2011, 29, 207–216. [Google Scholar]
- Mueller, T.C.; Senseman, S.A. Methods related to herbicide dissipation or degradation under field or laboratory conditions. Weed Sci. 2015, 63, 133–139. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 2, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Estatistical Computing; R Fundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Westgate, M.J. Revtools: An R package to support article screening for evidence synthesis. Res. Synth. Methods 2019, 10, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Feinerer, I.; Hornik, K.; Meyer, D. Text Mining Infrastructure in R. J. Stat. Softw. 2008, 25, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Richardson, G.M.; Bowers, J.; Woodill, A.J.; Barr, J.R.; Gawron, J.M.; Levine, R.A. Topic models: A tutorial with R. Int. J. Semant. Comput. 2014, 08, 85–98. [Google Scholar] [CrossRef]
- Robinson, D.; Hayes, A. Broom: Convert Statistical Analysis Objects into Tidy Tibbles, v0.5.4; [Open Source Package for R Software]. 2020. Available online: https://CRAN.R-project.org/package=broom (accessed on 15 May 2020).
- Fellows, I. Wordcloud: Word Clouds, v2.6; [Open Source Package for R Software]. 2018. Available online: https://CRAN.R-project.org/package=wordcloud (accessed on 15 May 2020).
- Lajeunesse, M.J. Facilitating systematic reviews, data extraction and meta-analysis with the metagear package for r. Methods Ecol. Evol. 2016, 7, 323–330. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Ialongo, C. Understanding the effect size and its measures. Biochem. Med. 2016, 26, 150–163. [Google Scholar] [CrossRef] [Green Version]
- Aloe, A.M.; Becker, B.J. An effect size for regression predictors in meta-analysis. J. Educ. Behav. Stat. 2012, 37, 278–297. [Google Scholar] [CrossRef]
- Su, W.; Hao, H.; Ding, M.; Wu, R.; Xu, H.; Xue, F.; Shen, C.; Sun, L.; Lu, C. Adsorption and degradation of imazapic in soils under different environmental conditions. PLoS ONE 2019, 14, e0219462. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.M.; Wang, H.L.; Fan, D.F. Biodegradation of imazapyr in typical soils in Zhejiang Province, China. J. Environ. Sci. 2005, 17, 593–597. [Google Scholar]
- Wu, H.; He, X.; Dong, H.; Zhou, Q.; Zhang, Y. Impact of microorganisms, humidity, and temperature on the enantioselective degradation of imazethapyr in two soils. Chirality 2017, 29, 348–357. [Google Scholar] [CrossRef]
- Flint, J.L.; Witt, W.W. Microbial degradation of imazaquin and imazethapyr. Weed Sci. 1997, 45, 586–591. [Google Scholar] [CrossRef]
- Avila, L.A.; Massey, J.H.; Senseman, S.A.; Armbrust, K.L.; Lancaster, S.R.; McCauley, G.N.; Chandler, J.M. Imazethapyr aqueous photolysis, reaction quantum yield, and hydroxyl radical rate constant. J. Agric. Food Chem. 2006, 54, 2635–2639. [Google Scholar] [CrossRef]
- Ramezani, M.; Oliver, D.P.; Kookana, R.S.; Gill, G.; Preston, C. Abiotic degradation (photodegradation and hydrolysis) of imidazolinone herbicides. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2008, 43, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Peterson, A.; Anderson, S.C.; Lass, R.; Johnson, M.; Nienow, A.M. Analysis of the photodegradation of the imidazolinone herbicides imazamox, imazapic, imazaquin, and imazamethabenz-methyl in aqueous solution. J. Agric. Food Chem. 2015, 63, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Curran, W.S.; Loux, M.M.; Liebl, R.A.; Simmons, F.W. Photolysis of imidazolinone herbicides in aqueous-solution and on soil. Weed Sci. 1992, 40, 143–148. [Google Scholar] [CrossRef]
- Buerge, I.J.; Baechli, A.; Kasteel, R.; Portmann, R.; Lopez-Cabeza, R.; Schwab, L.F.; Poiger, T. Behavior of the chiral herbicide imazamox in soils: pH-dependent, enantioselective degradation, formation and degradation of several chiral metabolites. Environ. Sci. Technol. 2019, 53, 5725–5732. [Google Scholar] [CrossRef]
- Oufqir, S.; Madani, E.M.; Belghiti, A.M.; Zrineh, A.; Azzouzi, E.M. Adsorption of imazethapyr on six agricultural soils of Morocco: Evaluation of the impact of soil properties. Arab. J. Chem. 2013. [Google Scholar] [CrossRef] [Green Version]
- Wauchope, R.D.; Yeh, S.; Linders, J.B.H.J.; Kloskowski, R.; Tanaka, K.; Rubin, B.; Katayama, A.; Kördel, W.; Gerstl, Z.; Lane, M. Pesticide soil sorption parameters: Theory, measurement, uses, limitations and reliability. Pest Manag. Sci. 2002, 58, 419–445. [Google Scholar] [CrossRef]
- Aichele, T.M.; Penner, D. Adsorption, desorption, and degradation of imidazolinones in soil. Weed Technol. 2005, 19, 154–159. [Google Scholar] [CrossRef]
- Gianelli, V.; Bedmar, F.; Costa, J. Persistence and sorption of imazapyr in three Argentinean soils. Environ. Toxicol. Chem. 2014, 33, 29–34. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Fan, D. Persistence and metabolism of imazapyr in four typical soils of Zhejiang Province (China). Int. J. Environ. Anal. Chem. 2005, 85, 99–109. [Google Scholar] [CrossRef]
- Lopez-Cabeza, R.; Gamiz, B.; Cornejo, J.; Celis, R. Behavior of the enantiomers of the herbicide imazaquin in agricultural soils under different application regimes. Geoderma 2017, 293, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Firmino, L.E.; Santos, L.D.; Ferreira, F.A.; Ferreira, L.R.; Tiburcio, R.A.S. Sorção do imazapyr em solos com diferentes texturas. Planta Daninha 2008, 26, 395–402. [Google Scholar] [CrossRef]
- Hamilton, N.E.; Ferry, M. ggtern: Ternary diagrams using ggplot2. J. Stat. Softw. 2018, 87. [Google Scholar] [CrossRef] [Green Version]
- Carter, A. Herbicide movement in soils: Principles, pathways and processes. Weed Res. 2000, 40, 113–122. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, X. Modification to imazaquin degradation in soil amended with farm manure. Soil Sediment Contam. 2007, 17, 41–52. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Celis, R.; Cox, L. Changes in sorption and bioavailability of herbicides in soil amended with fresh and aged biochar. Geoderma 2019, 337, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Pusino, A.; Petretto, S.; Gessa, C. Adsorption and desorption of imazapyr by soil. J. Agric. Food Chem. 1997, 45, 1012–1016. [Google Scholar] [CrossRef]
- Loux, M.M.; Reese, K.D. Effect of soil-ph on adsorption and persistence of imazaquin. Weed Sci. 1992, 40, 490–496. [Google Scholar] [CrossRef]
- Arantes, S.A.d.C.M.; Lavorenti, A.; Tornisielo, V.L. Efeito da calagem na mineralização de 14C-glifosato em solos. Ciênc. E Agrotecnol. 2011, 35, 234–241. [Google Scholar] [CrossRef]
- Ravelli, A.; Pantani, O.; Calamai, L.; Fusi, P. Rates of chlorsulfuron degradation in three Brazilian oxisols. Weed Res. 1997, 37, 51–59. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A.; Morgan, J.A.W.; Wright, D.J. Role of soil pH in the development of enhanced biodegradation of fenamiphos. Appl. Environ. Microbiol. 2003, 69, 7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Martínez, V.; Tabatabai, M.A. Enzyme activities in a limed agricultural soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Ismail, B.S.; Ahmad, A.R. Attenuation of the herbicidal activities of glufosinate-ammonium and imazapyr in 2 soils. Agric. Ecosyst. Environ. 1994, 47, 279–285. [Google Scholar] [CrossRef]
- Fageria, N.K.; Carvalho, G.D.; Santos, A.B.; Ferreira, E.P.B.; Knupp, A.M. Chemistry of lowland rice soils and nutrient availability. Commun. Soil Sci. Plant Anal. 2011, 42, 1913–1933. [Google Scholar] [CrossRef]
- Wang, X.D.; Wang, H.L.; Fan, D.F. Degradation and metabolism of imazapyr in soils under aerobic and anaerobic conditions. Int. J. Environ. Anal. Chem. 2006, 86, 541–551. [Google Scholar] [CrossRef]
- Morrica, P.; Giordano, A.; Seccia, S.; Ungaro, F.; Ventriglia, M. Degradation of imazosulfuron in soil. Pest Manag. Sci. 2001, 57, 360–365. [Google Scholar] [CrossRef]
- Accinelli, C.; Screpanti, C.; Vicari, A. Influence of flooding on the degradation of linuron, isoproturon and metolachlor in soil. Agron. Sustain. Dev. 2005, 25, 401–406. [Google Scholar] [CrossRef]
- Jabusch, T.W.; Tjeerdema, R.S. Microbial degradation of penoxsulam in flooded rice field soils. J. Agric. Food Chem. 2006, 54, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Heiser, J.W. Dissipation and Carryover of Imidazolinone Herbicides in Imidazolinone-Resistant Rice (Oryza Sativa); University of Missouri: Columbia, MO, USA, 2007. [Google Scholar]
- Junkes, G.V.; Camargo, E.; Avila, L.; Kemmerich, M.; Gehrke, V.R. Manejo intermitente da irrigação favorece a dissipação da mistura imazapyr + imazapic em solos de várzea. In Proceedings of the XI Congresso Brasileiro de Arroz Irrigado, Balneário Camboriú, Brazil, 13–18 August 2019. [Google Scholar]
- Shui-Ming, Z.; Duo-Sen, L.; Zong-Sheng, W.; Xing-Fa, M. A kinetic model describing the effect of temperature on the loss rate of pesticides in soil. Ecol. Model. 1993, 70, 115–125. [Google Scholar] [CrossRef]
- Hu, M.; Liu, K.; Qiu, J.; Zhang, H.; Li, X.; Zeng, D.; Tan, H. Behavior of imidazolinone herbicide enantiomers in earthworm-soil microcosms: Degradation and bioaccumulation. Sci. Total Environ. 2020, 707. [Google Scholar] [CrossRef] [PubMed]
- Vischetti, C.; Casucci, C.; Perucci, P. Relationship between changes of soil microbial biomass content and imazamox and benfluralin degradation. Biol. Fertil. Soils 2002, 35, 13–17. [Google Scholar] [CrossRef]
- Han, L.; Guo, B.; Feng, J.; Lu, X.; Lin, J.-M. Study on the enantioselective degradation of imazethapyr in soil by CE. Chromatographia 2008, 68, 1071–1073. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, L.; Liu, K.; Guo, F.; Gao, L.; Liu, W. Activity, toxicity, molecular docking, and environmental effects of three imidazolinone herbicides enantiomers. Sci. Total Environ. 2018, 622–623, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Hu, H.; Mao, Y.; Ma, J.; Zhang, A.; Liu, W.; Fu, Z. Enantioselective phytotoxicity of the herbicide imazethapyr in rice. Chemosphere 2009, 76, 885–892. [Google Scholar] [CrossRef]
- Stanley, J.K.; Brooks, B.W. Perspectives on ecological risk assessment of chiral compounds. Integr. Environ. Assess. Manag. Int. J. 2009, 5, 364–373. [Google Scholar] [CrossRef]
- Schreiber, F.; Scherner, A.; Massey, J.H.; Zanella, R.; Avila, L.A. Dissipation of clomazone, imazapyr, and imazapic herbicides in paddy water under two rice flood management regimes. Weed Technol. 2017, 31, 330–340. [Google Scholar] [CrossRef]
- Santos, F.M.; Marchesan, E.; Machado, S.L.O.; Avila, L.A.; Zanella, R.; Gonçalves, F.F. Persistência dos herbicidas imazethapyr e clomazone em lâmina de água do arroz irrigado. Planta Daninha 2008, 26, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Reimche, G.B.; Machado, S.L.O.; Oliveira, M.A.; Zanella, R.; Dressler, V.L.; Flores, E.M.M.; Goncalves, F.F.; Donato, F.F.; Nunes, M.A.G. Imazethapyr and imazapic, bispyribac-sodium and penoxsulam: Zooplankton and dissipation in subtropical rice paddy water. Sci. Total Environ. 2015, 514, 68–76. [Google Scholar] [CrossRef]
- Arroz Irrigado: Recomendações Técnicas da Pesquisa Para o Sul do Brasil; SOSBAI: Bento Gonçalves, RS, Brasil, 2014; Volume 30.
- Marchesan, E.; dos Santos, F.M.; Grohs, M.; de Avila, L.A.; Machado, S.L.O.; Senseman, S.A.; Massoni, P.F.S.; Sartori, G.M.S. Carryover of imazethapyr and imazapic to nontolerant rice. Weed Technol. 2010, 28, 6–10. [Google Scholar] [CrossRef]
- Avila, L.A.; Teló, G.M.; Ferreira, R.B.; Marchesan, E.; Machado, S.L.O.; Rossato, T.L.; Cezimbra, D.M.; Rigão, G., Jr. Retorno da produção de arroz irrigado com cultivares convencionais após o uso do sistema Clearfield®. Planta Daninha 2010, 28, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Agostinetto, D.; Fraga, D.S.; Vargas, L.; Oliveira, A.C.B.; Andres, A.; Villela, F.A. Response of soybean cultivars in rotation with irrigated rice crops cultivated in Clearfield® system. Planta Daninha 2018, 36. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Meteorologia. Banco de Dados Meteorológicos Para Ensino e Pesquisa. 2020. Available online: https://portal.inmet.gov.br/dadoshistoricos (accessed on 15 May 2020).
- IRGA. Boletim de Resultados da Lavoura—Safra 2018/19—Arroz Irrigado e Soja em Rotação. Available online: https://irga-admin.rs.gov.br/upload/arquivos/201909/05171808-relatorio-da-safra-2018-19-31-agosto-2019.pdf (accessed on 28 May 2020).
- Kraemer, A.F.; Marchesan, E.; Avila, L.A.; Machado, S.L.O.; Grohs, M.; Massoni, P.F.S.; Sartori, G.M.S. Persistência dos herbicidas imazethapyr e imazapic em solo de várzea sob diferentes sistemas de manejo. Planta Daninha 2009, 27, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Villa, S.C.C.; Marchezan, E.; Avila, L.A.; Massoni, P.F.S.; Telo, G.M.; Machado, S.L.O.; Camargo, E.R. Arroz tolerante a imidazolinonas: Controle do arroz-vermelho, fluxo gênico e efeito residual do herbicida em culturas sucessoras não-tolerantes. Planta Daninha 2006, 24, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Souto, K.M.; de Avila, L.A.; Cassol, G.V.; de Oliveira Machado, S.L.; Marchesan, E. Phytoremediation of lowland soil contaminated with a formulated mixture of Imazethapyr and Imazapic. Rev. Cienc. Agron. 2015, 46, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Souto, K.M.; Jacques, R.J.S.; Avila, L.A.d.; Machado, S.L.d.O.; Zanella, R.; Refatti, J.P. Biodegradação dos herbicidas imazetapir e imazapique em solo rizosférico de seis espécies vegetais. Ciência Rural 2013, 43, 1790–1796. [Google Scholar] [CrossRef] [Green Version]
- Galon, L.; Lima, A.M.; Guimarães, S.; Belarmino, J.G.; Burg, G.M.; Concenço, G.; Bastiani, M.O.; Beutler, A.N.; Zandona, R.R.; Radünz, A.L. Potential of plant species for bioremediation of soils applied with imidazolinone herbicides. Planta Daninha 2014, 32, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Souto, K.M.; Seminotti Jacques, R.J.; Zanella, R.; de Oliveira Machado, S.L.; Balbinot, A.; de Avila, L.A. Phytostimulation of lowland soil contaminated with imidazolinone herbicides. Int. J. Phytoremediat. 2020, 22, 774–780. [Google Scholar] [CrossRef]
- Boeni, M.; Anghinoni, I.; Junior, S.A.G.; Filho, B.D.O. Evolução da Fertilidade Dos Solos Cultivados Com Arroz Irrigado no Rio Grande do Sul; RS: Cachoeirinha, Brazil, 2010; p. 4. [Google Scholar]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehrke, V.R.; Fipke, M.V.; Avila, L.A.d.; Camargo, E.R. Understanding the Opportunities to Mitigate Carryover of Imidazolinone Herbicides in Lowland Rice. Agriculture 2021, 11, 299. https://doi.org/10.3390/agriculture11040299
Gehrke VR, Fipke MV, Avila LAd, Camargo ER. Understanding the Opportunities to Mitigate Carryover of Imidazolinone Herbicides in Lowland Rice. Agriculture. 2021; 11(4):299. https://doi.org/10.3390/agriculture11040299
Chicago/Turabian StyleGehrke, Vinicios Rafael, Marcus Vinicius Fipke, Luis Antonio de Avila, and Edinalvo Rabaioli Camargo. 2021. "Understanding the Opportunities to Mitigate Carryover of Imidazolinone Herbicides in Lowland Rice" Agriculture 11, no. 4: 299. https://doi.org/10.3390/agriculture11040299