Anaesthesia Management for Giant Intraabdominal Tumours: A Case Series Study
Abstract
:1. Background
2. Material and Methods
3. Results
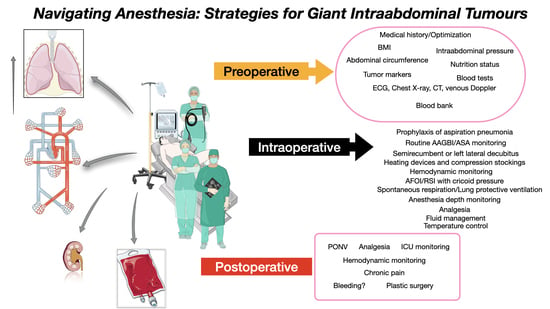
4. Discussions
- Adequate patient preparation (multidisciplinary approach; pharmacologic; nutritional);
- Book an ICU bed before surgery;
- Make sure there are blood products available;
- Warm the patient before induction of anaesthesia;
- Optimal patient positioning—left lateral decubitus/semirecumbent position;
- If possible, perform intubation while awake;
- Maintain initial spontaneous respirations until abdominal decompression;
- Advanced haemodynamic monitoring and goal-directed fluid therapy;
- Gradual aspiration of the fluid (if cystic)—0.5–1 L/min;
- Beware of postoperative intestinal distention.
- Studies comparing the management of anaesthesia in giant intraabdominal tumours are needed to help define the optimal strategy and establish a standard of care for an individualised approach.
- Complex respiratory and haemodynamic monitoring (CO, SV, SVV, and SVR) before, during, and after surgery could reveal major differences. Imagistical studies with complex measurements could help in defining exactly what organs are compromised due to the compression of the tumour, and different positioning and surgical approaches could be suggested.
- Improving the quality of life.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RPE | re-expansion pulmonary oedema |
| ICU | intensive care unit |
| CO | cardiac output |
| PPV | pulse pressure variation |
| SVV | stroke volume variation |
| SPI | Surgical Plethysmographic Index |
| TCI | target-controlled infusion |
| TAP | transversus abdominis plane |
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
- Wu, L.; Shi, S.; Sun, H.; Zhang, H. Tumor Size Is an Independent Prognostic Factor for Stage I Ovarian Clear Cell Carcinoma: A Large Retrospective Cohort Study of 1000 Patients. Front. Oncol. 2022, 12, 862944. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J. Size Matters for Sarcomas! Ann. R. Coll. Surg. Engl. 2006, 88, 519–524. [Google Scholar] [CrossRef]
- Govaerts, K.; Lurvink, R.; De Hingh, I.H.J.T.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Berger, J.S. Perioperative Cardiovascular Risk Assessment and Management for Noncardiac Surgery: A Review. JAMA 2020, 324, 279–290. [Google Scholar] [CrossRef]
- Bazurro, S.; Ball, L.; Pelosi, P. Perioperative management of obese patient. Curr. Opin. Crit. Care 2018, 24, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.K.; Garcia, L. Intraabdominal Hypertension, Abdominal Compartment Syndrome, and the Open Abdomen. Chest 2018, 153, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Sarani, B. Evaluation and management of intraabdominal hypertension. Curr. Opin. Crit. Care 2020, 26, 192–196. [Google Scholar] [CrossRef]
- Corp, A.; Thomas, C.; Adlam, M. The cardiovascular effects of positive pressure ventilation. BJA Educ. 2021, 21, 202–209. [Google Scholar] [CrossRef]
- Sohara, Y. Reexpansion pulmonary edema. Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia. 2008, 14, 205–209. [Google Scholar]
- Einenkel, J.; Alexander, H.; Schotte, D.; Stumpp, P.; Horn, L.C. Giant ovarian cysts: Is a pre- and intraoperative drainage an advisable procedure? Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2006, 16, 2039–2043. [Google Scholar] [CrossRef]
- Nishiyama, T.; Hanaoka, K. Same day drainage and removal of a giant ovarian cyst. Can. J. Anaesth. 1997, 44, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Kim, J.W.; Choe, B.H. A case of huge ovarian cyst of 21-year-old young woman. J. Obstet. Gynaecol. Res. 1999, 25, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.A.; Jaeger, M.T.; Siemens, D.R. Intra-operative inferior vena cava syndrome in a patient with autosomal dominant polycystic kidney disease. World J. Urol. 2006, 24, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Yanazume, Y.; Yoshinaga, M.; Yanazume, S.; Kamikihara, T.; Tokunaga, M.; Kamio, M.; Douchi, T. Giant ovarian cancer weighing 100 kg with poor prognosis. J. Obstet. Gynaecol. Res. 2007, 33, 91–94. [Google Scholar] [CrossRef]
- Ueda, S.; Yamada, Y.; Tsuji, Y.; Kawaguchi, R.; Haruta, S.; Shigetomi, H.; Kanayama, S.; Yoshida, S.; Sakata, M.; Sado, T.; et al. Giant abdominal tumor of the ovary. J. Obstet. Gynaecol. Res. 2008, 34, 108–111. [Google Scholar] [CrossRef]
- Fobe, D.; Vandervurst, T.; Vanhoutte, L. Giant ovarian cystadenoma weighing 59 kg. Gynecol. Surg. 2011, 8, 177–179. [Google Scholar] [CrossRef]
- Kincey, J.; Westin, S.N.; Zhao, B.; Curtis, M.G.; Ramondetta, L. Surgical removal of a gigantic abdominal mass: A multidisciplinary approach. Obstet. Gynecol. 2011, 117, 508–512. [Google Scholar] [CrossRef]
- Bamba, K.; Watanabe, T.; Kohno, T. Anesthetic management of a patient with a giant ovarian tumor containing 83 L of fluid. SpringerPlus 2013, 2, 487. [Google Scholar] [CrossRef]
- Madhu, Y.; Harish, K.; Gotam, P. Complete resection of a giant ovarian tumour. Gynecol. Oncol. Case Rep. 2013, 6, 4–6. [Google Scholar] [CrossRef]
- Ohashi, N.; Imai, H.; Tobita, T.; Ishii, H.; Baba, H. Anesthetic management in a patient with giant growing teratoma syndrome: A case report. J. Med. Case Rep. 2014, 8, 32. [Google Scholar] [CrossRef]
- Kb, N.; Pt, P.; Shivanna, S.; Cvr, M. Anaesthetic implications and management of a giant ovarian cyst. J. Clin. Diagn. Res. JCDR. 2014, 8, 170–171. [Google Scholar]
- Cîrstoiu, M.M.; Sajin, M.; Secară, D.C.; Munteanu, O.; Cîrstoiu, F.C. Giant ovarian mucinous cystadenoma with borderline areas: A case report. Rom. J. Morphol. Embryol. 2014, 55, 1443–1447. [Google Scholar]
- Feng, D.; Xu, F.; Wang, M.; Gu, X.; Ma, Z. Anesthetic management of a patient with giant retroperitoneal liposarcoma: Case report with literature review. Int. J. Clin. Exp. Med. 2015, 8, 19530–19534. [Google Scholar] [PubMed]
- Resection of a giant ovarian tumor the management challenge. MOJ Surg. 2015, 2, 00022. Available online: https://medcraveonline.com/MOJS/MOJS-02-00022.pdf (accessed on 15 December 2023).
- Akazawa, M.; Saito, T.; Nagayama, R.; Ariyoshi, K.; Okadome, M. Management of a Giant Ovarian Tumor More Than 30 kg: A Case Report and Review of the Literature. J. Gynecol. Surg. 2018, 34, 243–247. [Google Scholar] [CrossRef]
- Cai, S.; Dai, R.; Mi, J.; Wang, S.; Jiang, Y. Perioperative management of a patient with a giant ovarian tumor: A case report. Medicine 2020, 99, e22625. [Google Scholar] [CrossRef]
- Xiang, B.; Yi, M.; Yin, H.; Chen, R.; Yuan, F. Anesthesia management of an aged patient with giant abdominal tumor and large hiatal hernia: A case report and literature review. Front. Surg. 2022, 9, 921887. [Google Scholar] [CrossRef] [PubMed]
- Bojanic, M.; Radovanovic, D.; Zahorjanski, S.; Skoric-Jokic, S.; Protic, M. A patient with large retroperitoneal liposarcom—A challenge for an anesthesiologist. Arch. Oncol. 2022, 28, 28–31. [Google Scholar] [CrossRef]
- Yamochi, S.; Kinoshita, M.; Sawa, T. Anesthetic management of a severely obese patient (body mass index 70.1 kg/m2) undergoing giant ovarian tumor resection: A case report. J. Med. Case Rep. 2022, 16, 164. [Google Scholar] [CrossRef]
- Spohn, A.E. Multicystic ovarian tumor weighing 328 lb. Tex. Med. J. 1905, 1906, 273–274. [Google Scholar]
- Eames, D.H. Removal of 184 pound ovarian tumor, and observations regarding splanchnic shock. Am. J. Obstet. Gynecol. 1954, 67, 1358–1364. [Google Scholar] [CrossRef]
- O’Hanlan, K.A. Resection of a 303.2-pound ovarian tumor. Gynecol. Oncol. 1994, 54, 365–371. [Google Scholar] [CrossRef]
- Chao, A.; Chao, A.; Yen, Y.S.; Huang, C.H. Abdominal compartment syndrome secondary to ovarian mucinous cystadenoma. Obstet. Gynecol. 2004, 104, 1180–1182. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Balogh, Z.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Asciutto, G.; Mumme, A.; Marpe, B.; Hummel, T.; Asciutto, K.; Geier, B. Acute iliofemoral deep venous thrombosis due to giant ovarian tumor: Report of a hybrid treatment. VASA Z. Gefasskrankh. 2008, 37, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Alhomary, M.; Ramadan, E.; Curran, E.; Walsh, S.R. Videolaryngoscopy vs. fibreoptic bronchoscopy for awake tracheal intubation: A systematic review and meta-analysis. Anaesthesia 2018, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Cabrini, L.; Redaelli, M.B.; Ball, L.; Filippini, M.; Fominskiy, E.; Pintaudi, M.; Putzu, A.; Votta, C.D.; Sorbello, M.; Antonelli, M.; et al. Awake Fiberoptic Intubation Protocols in the Operating Room for Anticipated Difficult Airway: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Obstet. Anesthesia Dig. 2019, 128, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Klucka, J.; Kosinova, M.; Zacharowski, K.; De Hert, S.; Kratochvil, M.; Toukalkova, M.; Stoudek, R.; Zelinkova, H.; Stourac, P. Rapid sequence induction: An international survey. Eur. J. Anaesthesiol. 2020, 37, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, T.; Hammond, B.; Talarek, C.; Sinha, A.C.; Brister, N.W. Anesthetic Management for Paraesophageal Hernia Repair. Thorac. Surg. Clin. 2019, 29, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Miller, C.; Bräuer, A.; Wallner, B.; Bock, M.; Paal, P. Perioperative Hypothermia—A Narrative Review. Int. J. Environ. Res. Public. Health 2021, 18, 8749. [Google Scholar] [CrossRef]
- Shinohara, H.; Ishii, H.; Kakuyama, M.; Fukuda, K. Morbidly obese patient with a huge ovarian tumor who was intubated while awake using airway scope in lateral decubitus position. Masui. Jpn. J. Anesthesiol. 2010, 59, 625–628. [Google Scholar]
- Silva, P.L.; Rocco, P.R.M. The basics of respiratory mechanics: Ventilator-derived parameters. Ann. Transl. Med. 2018, 6, 376. [Google Scholar] [CrossRef]
- Miyawaki, J.; Shono, S.; Goto, H.; Beppu, R.; Higa, K. Anesthetic management of a patient with a huge ovarian tumor. Masui. Jpn. J. Anesthesiol. 2000, 49, 552–554. [Google Scholar]
- Massoth, C.; Chappell, D.; Kranke, P.; Wenk, M. Supine hypotensive syndrome of pregnancy: A review of current knowledge. Obstet. Anesthesia Dig. 2023, 43, 7. [Google Scholar] [CrossRef]
- Weinberg, L.; Fink, M.; Tan, C.O.; Miles, L.F. Haemodynamic and respiratory changes following surgical resection of a giant ovarian cystadenoma. BMJ Case Rep. 2019, 12, e232139. [Google Scholar] [CrossRef] [PubMed]
- Mahfood, S.; Hix, W.R.; Aaron, B.L.; Blaes, P.; Watson, D.C. Reexpansion pulmonary edema. Ann. Thorac. Surg. 1988, 45, 340–345. [Google Scholar] [CrossRef]
- Komatsu, T.; Shibata, S.; Seo, R.; Tomii, K.; Ishihara, K.; Hayashi, T.; Takahashi, Y. Unilateral re-expansion pulmonary edema following treatment of pneumothorax with exceptionally massive sputum production, followed by circulatory collapse. Can. Respir. J. 2010, 17, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.W.; Bailey, J.W.; Wilkinson, P.; Rudd, R.M. Reexpansion pulmonary edema. Chest 1992, 102, 1920–1921. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Jonkman, A.H.; Dianti, J.; Vaporidi, K.; Beitler, J.R.; Patel, B.K.; Yoshida, T.; Jaber, S.; Dres, M.; Mauri, T.; et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: Avoiding insufficient and excessive effort. Intensiv. Care Med. 2020, 46, 2314–2326. [Google Scholar] [CrossRef]
- Rami Reddy, S.R.; Cappell, M.S. A Systematic Review of the Clinical Presentation, Diagnosis, and Treatment of Small Bowel Obstruction. Curr. Gastroenterol. Rep. 2017, 19, 28. [Google Scholar] [CrossRef]
- Symmonds, R.E.; Spraitz, A.F.; Koelsche, G.A. Large ovarian tumor. Report of a case. Obstet Gynecol. 1963, 22, 473–477. [Google Scholar]
- Usubiaga, J.E.; Moya, F.; Usubiaga, L.E. Effect of thoracic and abdominal pressure changes on the epidural space pressure. Br. J. Anaesth. 1967, 39, 612–618. [Google Scholar] [CrossRef]
- Suehiro, K.; Tanaka, K.; Matsuura, T.; Funao, T.; Yamada, T.; Mori, T.; Nishikawa, K. The Vigileo-FloTracTM system: Arterial waveform analysis for measuring cardiac output and predicting fluid responsiveness: A clinical review. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-F.; Liu, F.-C.; Yu, H.-P. FloTrac/Vigileo system monitoring in acute-care surgery: Current and future trends. Expert Rev. Med. Devices 2013, 10, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Kostyk, P.; Francois, K.; Salik, I. Airway Anesthesia for Awake Tracheal Intubation: A Review of the Literature. Cureus 2021, 13, e16315. [Google Scholar] [CrossRef] [PubMed]





| No./ Year/ Reference | Age | Sex | Weight | Height (m) | BMI | IBW (kg) | Abdominal Circumference (cm) | Pathology | Medical History | Weight of Tumour (kg) | Postoperative Weight (kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 1997 [12] | 58 | female | 107.6 | 1.5 | 48 | 43 | 163.5 | mucinous cystadenoma of ovary | - | 47 | - |
| 2 1999 [13] | 21 | female | 103 | 1.53 | 44 | 46 | 160 | mucinous borderline tumour of the intestinal type confined to the right ovary | malnutrition, dyspnea, amenorrhea | 62 | 44 |
| 3 2006 [11] | 57 | female | 102 | 1.65 | 37 | 57 | 150 | mucinous cystadenoma of ovary | malnutrition, dyspnea | 49 | 53 |
| 4 2006 [14] | 42 | male | - | - | - | - | - | autosomal dominant polycystic kidney disease | hypertension, CKD V | 10.5 | - |
| 5 2007 [15] | 30 | female | 148 | 1.63 | 56 | 55 | 198 | mucinous cystadenoma of ovary | dyspnea | 100 | - |
| 6 2008 [16] | 34 | female | 125 | 1.5 | 56 | 43 | 193 | serous cystadenoma of ovary | malnutrition, amenorrhea | 86.5 | 39.3 |
| 7 2010 [17] | 24 | female | 122 | 1.65 | 45 | 57 | - | giant mucosal-serosal cystadenoma | dyspnea, congenital bilateral clubfoot | 59 | - |
| 8 2011 [18] | 45 | female | - | - | - | - | 140 | serous cystadenomas of paraovarian or paratubal origin | - | - | - |
| 9 2013 [19] | 59 | female | 146 | 1.54 | 62 | 47 | 194 | benign ovarian cyst | - | 100 | 50 |
| 10 2013 [20] | 55 | female | 90 | - | - | - | 190 | mucinous cystadenoma of ovary | - | 56.95 | - |
| 11 2014 [21] | 30 | female | 57 | 1.61 | 22 | 53 | - | mature teratoma without malignancy | ovarian germ cell tumor at age 12 | 10.5 | - |
| 12 2014 [22] | 50 | female | 90 | - | - | - | 170 | mucinous cystadenoma of ovary | - | 56.9 | 32 |
| 13 2014 [23] | 44 | female | 88 | - | - | - | - | enteric type of multilocular mucinous ovarian cyst adenoma | - | 30 | 57 |
| 14 2015 [24] | 66 | male | - | - | 26 | - | - | liposarcoma-retroperitoneal | - | 4.5 | - |
| 15 2015 [25] | 20 | female | 100 | - | - | - | 161 | giant ovarian tumour | pneumonia, obstructive pyelonephrosis and hydroureter | - | - |
| 16 2018 [26] | 37 | female | 154.3 | 1.66 | 56 | 58 | 178 | mucinous borderline tumour | asthma | 57 | 85 |
| 17 2020 [27] | 66 | female | 68 | 1.61 | 26 | 53 | 110 | mucinous cystadenoma of ovary | - | - | - |
| 18 2022 [28] | 82 | female | 41.5 | 1.55 | 17 | 48 | - | spindle cell tumor (desmoid type fibromatosis) | hypertension, valvular heart disease | - | - |
| 19 2022 [29] | 57 | male | 78 | 1.74 | 26 | 70 | 136 | liposarcoma (retroperitoneal) | hypertension, smoker and COPD | 30.4 | - |
| 20 2022 [30] | 46 | female | 193.2 | 1.66 | 70 | 58 | - | giant ovarian tumour | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grăjdieru, O.; Petrișor, C.; Bodolea, C.; Tomuleasa, C.; Constantinescu, C. Anaesthesia Management for Giant Intraabdominal Tumours: A Case Series Study. J. Clin. Med. 2024, 13, 1321. https://doi.org/10.3390/jcm13051321
Grăjdieru O, Petrișor C, Bodolea C, Tomuleasa C, Constantinescu C. Anaesthesia Management for Giant Intraabdominal Tumours: A Case Series Study. Journal of Clinical Medicine. 2024; 13(5):1321. https://doi.org/10.3390/jcm13051321
Chicago/Turabian StyleGrăjdieru, Olga, Cristina Petrișor, Constantin Bodolea, Ciprian Tomuleasa, and Cătălin Constantinescu. 2024. "Anaesthesia Management for Giant Intraabdominal Tumours: A Case Series Study" Journal of Clinical Medicine 13, no. 5: 1321. https://doi.org/10.3390/jcm13051321