Total Muscle Area and Visceral Adipose Tissue Measurements for Frailty Assessment in TAVR Patients
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Transcatheter Aortic Valve Replacement
2.3. CT Assessment
2.4. Data Collection
2.5. Endpoints
2.6. Statistical Analysis
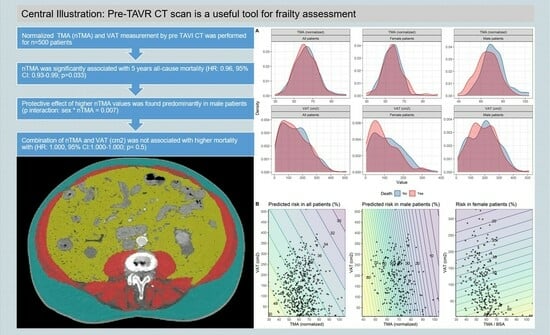
3. Results
- (I)
- Muscle mass:
- (II)
- Fat mass:
- (III)
- Interaction of Muscle and Fat mass:
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAVR | Transcatheter aortic valve replacement |
| AS | Aortic valve stenosis |
| TMA | Total muscle area |
| nTMA | Normalized total muscle area |
| PMA | Psoas muscle area |
| VAT | Visceral adipose tissue |
References
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Kondapally Seshasai, S.R.; Firoozi, S.; Brecker, S.J. Transcatheter aortic valve replacement versus surgery for symptomatic severe aortic stenosis: A reconstructed individual patient data meta-analysis. Catheter. Cardiovasc. Interv. 2020, 96, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Schenker, C.; Wertli, M.M.; Räber, L.; Haynes, A.G.; Chiolero, A.; Rodondi, N.; Panczak, R.; Aujesky, D. Regional variation and temporal trends in transcatheter and surgical aortic valve replacement in Switzerland: A population-based small area analysis. PLoS ONE 2024, 19, e0296055. [Google Scholar] [CrossRef]
- Grossman, Y.; Barbash, I.M.; Fefer, P.; Goldenberg, I.; Berkovitch, A.; Regev, E.; Fink, N.; Ben-Zekry, S.; Brodov, Y.; Kogan, A.; et al. Addition of albumin to Traditional Risk Score Improved Prediction of Mortality in Individuals Undergoing Transcatheter Aortic Valve Replacement. J. Am. Geriatr. Soc. 2017, 65, 2413–2417. [Google Scholar] [CrossRef]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefèvre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Søndergaard, L.; Kirk, B.H.; Jørgensen, T.H. Frailty: An Important Measure in Patients Considered for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 404–406. [Google Scholar] [CrossRef]
- Ilic, I.; Faron, A.; Heimann, M.; Potthoff, A.-L.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; et al. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers 2021, 13, 3353. [Google Scholar] [CrossRef]
- Bentov, I.; Kaplan, S.J.; Pham, T.N.; Reed, M.J. Frailty assessment: From clinical to radiological tools. Br. J. Anaesth. 2019, 123, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Flexman, A.M.; Street, J.; Charest-Morin, R. The impact of frailty and sarcopenia on patient outcomes after complex spine surgery. Curr. Opin. Anaesthesiol. 2019, 32, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, K.; Witowski, J.; Tylec, P.; Grochowska, A.; Przytuła, N.; Lis, M.; Pędziwiatr, M.; Rubinkiewicz, M. Radiological Features for Frailty Assessment in Patients Requiring Emergency Laparotomy. J. Clin. Med. 2022, 11, 5365. [Google Scholar] [CrossRef]
- de Bree, R.; Meerkerk, C.D.A.; Halmos, G.B.; Mäkitie, A.A.; Homma, A.; Rodrigo, J.P.; López, F.; Takes, R.P.; Vermorken, J.B.; Ferlito, A. Measurement of Sarcopenia in Head and Neck Cancer Patients and Its Association with Frailty. Front. Oncol. 2022, 12, 884988. [Google Scholar] [CrossRef]
- Okamura, H.; Kimura, N.; Mieno, M.; Yuri, K.; Yamaguchi, A. Preoperative sarcopenia is associated with late mortality after off-pump coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2020, 58, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Canales, C.; Mazor, E.; Coy, H.; Grogan, T.R.; Duval, V.; Raman, S.; Cannesson, M.; Singh, S.P. Preoperative Point-of-Care Ultrasound to Identify Frailty and Predict Postoperative Outcomes: A Diagnostic Accuracy Study. Anesthesiology 2022, 136, 268–278. [Google Scholar] [CrossRef]
- Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, W.-Y.; Lin, C.-H.; Chang, C.-K.; Li, T.-C.; Lin, C.-C. Sarcopenia Defined by Combining Height- and Weight-Adjusted Skeletal Muscle Indices is Closely Associated with Poor Physical Performance. J. Aging Phys. Act. 2015, 23, 597–606. [Google Scholar] [CrossRef]
- McIsaac, D.I. Preoperative Frailty Assessment: An Opportunity to Add Value to Perioperative Care. Anesthesiology 2022, 136, 255–257. [Google Scholar] [CrossRef]
- Mamane, S.; Mullie, L.; Piazza, N.; Martucci, G.; Morais, J.; Vigano, A.; Levental, M.; Nelson, K.; Lange, R.; Afilalo, J. Psoas Muscle Area and All-Cause Mortality After Transcatheter Aortic Valve Replacement: The Montreal-Munich Study. Can. J. Cardiol. 2016, 32, 177–182. [Google Scholar] [CrossRef]
- Saji, M.; Lim, D.S.; Ragosta, M.; LaPar, D.J.; Downs, E.; Ghanta, R.K.; Kern, J.A.; Dent, J.M.; Ailawadi, G. Usefulness of Psoas Muscle Area to Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2016, 118, 251–257. [Google Scholar] [CrossRef]
- Mok, M.; Allende, R.; Leipsic, J.; Altisent, O.A.-J.; del Trigo, M.; Campelo-Parada, F.; DeLarochellière, R.; Dumont, E.; Doyle, D.; Côté, M.; et al. Prognostic Value of Fat Mass and Skeletal Muscle Mass Determined by Computed Tomography in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2016, 117, 828–833. [Google Scholar] [CrossRef]
- Okuno, T.; Koseki, K.; Nakanishi, T.; Ninomiya, K.; Tomii, D.; Tanaka, T.; Sato, Y.; Osanai, A.; Sato, K.; Koike, H.; et al. Prognostic Impact of Computed Tomography-Derived Abdominal Fat Area on Transcatheter Aortic Valve Implantation. Circ. J. 2018, 82, 3082–3089. [Google Scholar] [CrossRef]
- Stortecky, S.; Franzone, A.; Heg, D.; Tueller, D.; Noble, S.; Pilgrim, T.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Nietlispach, F.; et al. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: A SwissTAVI Registry analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 242–251. [Google Scholar] [CrossRef]
- Tomii, D.; Okuno, T.; Heg, D.; Lanz, J.; Praz, F.; Stortecky, S.; Windecker, S.; Pilgrim, T. Validation of the VARC-3 Technical Success Definition in Patients Undergoing TAVR. JACC Cardiovasc. Interv. 2022, 15, 353–364. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 13 July 2023).
- Rodríguez, A.J.; Scott, D.; Hodge, A.; English, D.R.; Giles, G.G.; Ebeling, P.R. Associations between hip bone mineral density, aortic calcification and cardiac workload in community-dwelling older Australians. Osteoporos. Int. 2017, 28, 2239–2245. [Google Scholar] [CrossRef]
- Shi, L.; Yu, X.; Pang, Q.; Chen, X.; Wang, C. The associations between bone mineral density and long-term risks of cardiovascular disease, cancer, and all-cause mortality. Front. Endocrinol. 2022, 13, 938399. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Yamamoto, M.; Yamada, S.; Kobayashi, T.; Morita, S.; Kagase, A.; Tokuda, T.; Shimura, T.; Tsunaki, T.; Tada, N.; et al. Clinical Outcomes of Subcutaneous and Visceral Adipose Tissue Characteristics Assessed in Patients Underwent Transcatheter Aortic Valve Replacement. CJC Open 2021, 3, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bocca, G.; Mastoridis, S.; Yeung, T.; James, D.R.C.; Cunningham, C. Visceral-to-subcutaneous fat ratio exhibits strongest association with early post-operative outcomes in patients undergoing surgery for advanced rectal cancer. Int. J. Color. Dis. 2022, 37, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- He, A.Q.; Li, C.Q.; Zhang, Q.; Liu, T.; Liu, J.; Liu, G. Visceral-to-Subcutaneous Fat Ratio Is a Potential Predictor of Postoperative Complications in Colorectal Cancer. Med. Sci. Monit. 2021, 27, e930329. [Google Scholar] [CrossRef] [PubMed]
- Pernik, M.N.; Hicks, W.H.; Akbik, O.S.; Nguyen, M.L.; Luu, I.; Traylor, J.I.; Deme, P.R.; Dosselman, L.J.; Hall, K.; Wingfield, S.A.; et al. Psoas Muscle Index as a Predictor of Perioperative Outcomes in Geriatric Patients Undergoing Spine Surgery. Global Spine J. 2022, 13, 2016–2024. [Google Scholar] [CrossRef]
- Miao, S.L.; Ye, X.N.; Lin, T.T.; Qiu, Y.-H.; Huang, J.-Y.; Zheng, X.-W.; Chen, F.-F. The psoas muscle density as a predictor of postoperative complications and 30-day mortality for acute mesenteric ischemia patients. Abdom. Radiol. 2022, 47, 1644–1653. [Google Scholar] [CrossRef]
- Batista, A.F.R.; Petty, D.; Fairhurst, C.; Davies, S. Psoas muscle mass index as a predictor of long-term mortality and severity of complications after major intra-abdominal colorectal surgery—A retrospective analysis. J. Clin. Anesth. 2023, 84, 110995. [Google Scholar] [CrossRef]
- Balsam, L.B. Psoas muscle area: A new standard for frailty assessment in cardiac surgery? J. Thorac. Dis. 2018, 10 (Suppl. 33), S3846–S3849. [Google Scholar] [CrossRef] [PubMed]
- Paknikar, R.; Friedman, J.; Cron, D.; Deeb, G.M.; Chetcuti, S.; Grossman, P.M.; Wang, S.; Englesbe, M.; Patel, H.J. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2016, 151, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.; Ades, M.; Mullie, L.; Trnkus, A.; Morin, J.-F.; Langlois, Y.; Ma, F.; Levental, M.; Morais, J.A.; Afilalo, J. Psoas Muscle Area and Length of Stay in Older Adults Undergoing Cardiac Operations. Ann. Thorac. Surg. 2017, 103, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B.; Mehaffey, J.H.; Charles, E.J.; Kern, J.A.; Lim, D.S.; Teman, N.R.; Ailawadi, G. Psoas Muscle Size Predicts Risk-Adjusted Outcomes After Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 39–45. [Google Scholar] [CrossRef] [PubMed]




| All Patients n = 584 | |
|---|---|
| Gender: | |
| Male | 280 (47.9%) |
| Female | 304 (52.1%) |
| Age (years) | 82.8 [79.0; 86.5] |
| Height (cm) | 165 [158; 172] |
| Weight (kg) | 71.0 [62.0; 83.0] |
| Body mass index (kg/m2) | 25.6 [22.7; 29.7] |
| Diabetes mellitus [Yes] | 156 (26.7%) |
| Arterial hypertension [Yes] | 506 (86.6%) |
| Dyslipidemia [Yes] | 391 (67.0%) |
| Chronic obstructive pulmonary disease [Yes] | 61 (10.4%) |
| History of cerebrovascular accident [Yes] | 71 (12.2%) |
| Transient ischemic attack [Yes] | 31 (5.31%) |
| Coronary artery disease [Yes] | 346 (59.2%) |
| History of myocardial infarction [Yes] | 87 (14.9%) |
| Atrial fibrillation [Yes] | 203 (34.8%) |
| Peripheral artery disease [Yes] | 69 (11.8%) |
| History of cardiac surgery [Yes] | 59 (10.1%) |
| Dyspnea [Yes] | 578 (99.1%) |
| Body surface area (m2, Haycock) | 1.81 [1.67; 1.99] |
| Visceral adipose tissue (cm2) | 134 [68.5; 216] |
| Total muscle area (cm2) | 110 [93.4; 131] |
| Total muscle area normalized by body surface area (-) | 62.3 [54.5; 70.1] |
| Subcutaneous adipose tissue (cm2) | 148 [106; 207] |
| Creatinine (µmol/L) | 95.5 [77.0; 120] |
| Brain natriuretic peptide (pg/mL) | 257 [108; 625] |
| Albumin (g/L) | 34.0 [32.0; 36.0] |
| Mean gradient of the aortic valve (mmHg) | 39.0 [28.0; 47.0] |
| Peak gradient of the aortic valve (mmHg) | 63.0 [45.0; 78.0] |
| Aortic valve area (cm2) | 0.70 [0.60; 0.90] |
| Indexed aortic valve area (cm2) | 0.27 [0.21; 0.32] |
| Left ventricular ejection fraction (LVEF) (%) | 60.0 [50.0; 65.0] |
| Logistic Euro Score | 9.18 [5.83; 17.7] |
| Linear Euro Score | 8.00 [6.00; 10.0] |
| Euro Score-II | 3.67 [2.24; 6.66] |
| STS predicted risk of mortality | 4.12 [2.90; 6.31] |
| Cox Regression | |||
|---|---|---|---|
| Characteristic | HR | 95% CI | p-Value |
| Age (years) | 1.023 | 0.998, 1.049 | p = 0.069 |
| Body mass index (kg/m2) | 1.005 | 0.97, 1.046 | p = 0.8 |
| Sex: | |||
| Female | 0.036 | 0.003, 0.396 | p = 0.007 |
| TMA (normalized) | 0.96 | 0.927, 0.997 | p = 0.033 |
| VAT (cm2) | 1.002 | 0.99, 1.015 | p = 0.7 |
| Sex × TMA (normalized) | |||
| Female × TMA (normalized) | 1.048 | 1.013, 1.084 | p = 0.007 |
| Sex × VAT | |||
| Female × VAT (cm2) | 0.997 | 0.99, 1.001 | p = 0.2 |
| TMA (normalized) × VAT (cm2) | 0.9999 | 0.9998, 1. | P = 0.5 |
| Female Patients | Male Patients | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR 1 | 95% CI 1 | p-Value | HR 1 | 95% CI 1 | p-Value |
| Age (years) | 1.029 | 0.99, 1.070 | p = 0.14 | 1.018 | 0.99, 1.052 | p = 0.3 |
| BMI (kg/m2) | 1.003 | 0.948, 1.061 | p = >0.9 | 1.006 | 0.95, 1.065 | p = 0.8 |
| TMA (normalized) | 1.009 | 0.98, 1.038 | p = 0.6 | 0.96 | 0.917, 0.999 | p = 0.044 |
| VAT (cm2) | 1.001 | 0.98, 1.019 | p = >0.9 | 1.001 | 0.99, 1.017 | p = 0.9 |
| TMA (normalized) × VAT (cm2) | 0.9999 | 0.9996, 1. | p = 0.6 | 0. | 0.9997, 1. | p = 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, C.; Rothenbühler, C.F.; Huber, M.; Schweizer, M.; Todorski, I.; Gloor, D.A.; Windecker, S.; Lanz, J.; Stortecky, S.; Pilgrim, T.; et al. Total Muscle Area and Visceral Adipose Tissue Measurements for Frailty Assessment in TAVR Patients. J. Clin. Med. 2024, 13, 1322. https://doi.org/10.3390/jcm13051322
Demirel C, Rothenbühler CF, Huber M, Schweizer M, Todorski I, Gloor DA, Windecker S, Lanz J, Stortecky S, Pilgrim T, et al. Total Muscle Area and Visceral Adipose Tissue Measurements for Frailty Assessment in TAVR Patients. Journal of Clinical Medicine. 2024; 13(5):1322. https://doi.org/10.3390/jcm13051322
Chicago/Turabian StyleDemirel, Caglayan, Christoph Fritz Rothenbühler, Markus Huber, Michelle Schweizer, Inga Todorski, David Alexander Gloor, Stephan Windecker, Jonas Lanz, Stefan Stortecky, Thomas Pilgrim, and et al. 2024. "Total Muscle Area and Visceral Adipose Tissue Measurements for Frailty Assessment in TAVR Patients" Journal of Clinical Medicine 13, no. 5: 1322. https://doi.org/10.3390/jcm13051322