Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical Data
2.3. Doppler Echocardiography
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. One-Year and Late Clinical Outcomes
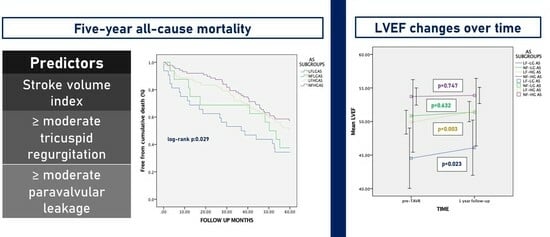
3.2. Predictors of Late Global Mortality
3.3. Impact of Pre-Tavi Flow on Late Outcomes
3.4. Changes in LVEF over Time
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| AV | Aortic valve |
| AVA | Aortic valve area |
| CABG | Coronary artery bypass graft |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CV | Cardiovascular |
| DSE | Dobutamine stress echocardiography |
| F | Flow |
| HG | High gradient |
| LF-LG | Low flow-low gradient |
| LVEF | Left ventricular ejection fraction |
| MACCE | Major adverse cardiovascular and cerebrovascular events |
| MG | Mean gradient |
| MI | Myocardial infarction |
| MR | Mitral regurgitation |
| MSCT | Multi-slice computed tomography |
| PAD | Peripheral artery disease |
| PASP | Pulmonary artery systolic pressure |
| PCI | Percutaneous coronary intervention |
| PVL | Paravalvular leak |
| SAVR | Surgical aortic valve replacement |
| SVi | Stroke volume indexed |
| TAVI | Transcatheter aortic valve implantation |
| TR | Tricuspid regurgitation |
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Dumesnil, J.G.; Capoulade, R.; Mathieu, P.; Sénéchal, M.; Pibarot, P. Outcome of Patients with Aortic Stenosis, Small Valve Area, and Low-Flow, Low-Gradient Despite Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2012, 60, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Lauten, J.; Rost, C.; Breithardt, O.A.; Seligmann, C.; Klinghammer, L.; Daniel, W.G.; Flachskampf, F.A. Invasive Hemodynamic Characteristics of Low Gradient Severe Aortic Stenosis Despite Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2013, 61, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, Z.; Dumesnil, J.G.; Bogaty, P.; Pibarot, P. Paradoxical Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Preserved Ejection Fraction Is Associated with Higher Afterload and Reduced Survival. Circulation 2007, 115, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Dumesnil, J.G.; Pibarot, P. Low-flow, low-gradient severe aortic stenosis in patients with normal ejection fraction. Curr. Opin. Cardiol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Low-Flow, Low-Gradient Aortic Stenosis with Normal and Depressed Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2012, 60, 1845–1853. [Google Scholar] [CrossRef]
- Kulik, A.; Kapila, V.; Mesana, T.G.; Ruel, M.; Burwash, I.G. Long-Term Outcomes after Valve Replacement for Low-Gradient Aortic Stenosis. Circulation 2006, 114, e628. [Google Scholar] [CrossRef]
- Connolly, H.M.; Oh, J.K.; Schaff, H.V.; Roger, V.L.; Osborn, S.L.; Hodge, D.O.; Tajik, A.J. Severe Aortic Stenosis with Low Transvalvular Gradient and Severe Left Ventricular Dysfunction. Circulation 2000, 101, 1940–1946. [Google Scholar] [CrossRef]
- Monin, J.-L.; Quéré, J.-P.; Monchi, M.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Low-Gradient Aortic Stenosis. Circulation 2003, 108, 319–324. [Google Scholar] [CrossRef]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.-P.; Neumann, F.-J.; Jander, N. Inconsistent grading of aortic valve stenosis by current guidelines: Haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Dumesnil, J.G.; Pibarot, P.; Carabello, B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: Implications for diagnosis and treatment. Eur. Heart J. 2010, 31, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Barasch, E.; Fan, D.; O Chukwu, E.; Han, J.; Passick, M.; Petillo, F.; Norales, A.; Reichek, N. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: Pathophysiologic and prognostic insights. J. Heart Valve Dis. 2008, 17, 81–88. [Google Scholar] [PubMed]
- Adda, J.; Mielot, C.; Giorgi, R.; Cransac, F.; Zirphile, X.; Donal, E.; Sportouch-Dukhan, C.; Réant, P.; Laffitte, S.; Cade, S.; et al. Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Normal Ejection Fraction Is Associated with Severe Left Ventricular Dysfunction as Assessed by Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging 2012, 5, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.J.; Lauer, M.S.; Bashir, M.; Afridi, I.; Blackstone, E.H.; Stewart, W.J.; McCarthy, P.M.; Thomas, J.D.; Asher, C.R. Survival after aortic valve replacement forsevere aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J. Am. Coll. Cardiol. 2002, 39, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Levy, F.; Laurent, M.; Monin, J.L.; Maillet, J.M.; Pasquet, A.; Le Tourneau, T.; Petit-Eisenmann, H.; Gori, M.; Jobic, Y.; Bauer, F.; et al. Aortic Valve Replacement for Low-Flow/Low-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2008, 51, 1466–1472. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Lévy, F.; Rusinaru, D.; Guéret, P.; Petit-Eisenmann, H.; Baleynaud, S.; Jobic, Y.; Adams, C.; Lelong, B.; Pasquet, A.; et al. Outcome after Aortic Valve Replacement for Low-Flow/Low-Gradient Aortic Stenosis without Contractile Reserve on Dobutamine Stress Echocardiography. J. Am. Coll. Cardiol. 2009, 53, 1865–1873. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Salaun, E.; Dahou, A.; Avenatti, E.; Guzzetti, E.; Annabi, M.-S.; Toubal, O.; Bernier, M.; Beaudoin, J.; Ong, G.; et al. Echocardiographic Results of Transcatheter versus Surgical Aortic Valve Replacement in Low-Risk Patients. Circulation 2020, 141, 1527–1537. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Saybolt, M.D.; Fiorilli, P.N.; Gertz, Z.M.; Herrmann, H.C. Low-Flow Severe Aortic Stenosis. Circ. Cardiovasc. Interv. 2017, 10, e004838. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, M.; Lindstaedt, M.; Bojara, W.; Ewers, A.; Mügge, A. Clinical outcome of transcatheter aortic valve implantation in patients with low-flow, low gradient aortic stenosis. Catheter. Cardiovasc. Interv. 2012, 79, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Lauten, A.; Zahn, R.; Horack, M.; Sievert, H.; Linke, A.; Ferrari, M.; Harnath, A.; Grube, E.; Gerckens, U.; Kuck, K.-H.; et al. Transcatheter Aortic Valve Implantation in Patients with Low-Flow, Low-Gradient Aortic Stenosis. JACC Cardiovasc. Interv. 2012, 5, 552–559. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.J.; Stortecky, S.; Heg, D.; Pilgrim, T.; Hosek, N.; Buellesfeld, L.; Khattab, A.A.; Nietlispach, F.; Moschovitis, A.; Zanchin, T.; et al. Clinical outcomes of patients with low-flow, low-gradient, severe aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur. Heart J. 2013, 34, 3437–3450. [Google Scholar] [CrossRef] [PubMed]
- Elhmidi, Y.; Piazza, N.; Krane, M.; Deutsch, M.; Mazzitelli, D.; Lange, R.; Bleiziffer, S. Clinical presentation and outcomes after transcatheter aortic valve implantation in patients with low flow/low gradient severe aortic stenosis. Catheter. Cardiovasc. Interv. 2014, 84, 283–290. [Google Scholar] [CrossRef]
- Kamperidis, V.; Joyce, E.; Debonnaire, P.; Katsanos, S.; van Rosendael, P.J.; van der Kley, F.; Sianos, G.; Bax, J.J.; Marsan, N.A.; Delgado, V. Left Ventricular Functional Recovery and Remodeling in Low-Flow Low-Gradient Severe Aortic Stenosis after Transcatheter Aortic Valve Implantation. J. Am. Soc. Echocardiogr. 2014, 27, 817–825. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Pibarot, P.; Hueter, I.; Gertz, Z.M.; Stewart, W.J.; Kapadia, S.; Tuzcu, E.M.; Babaliaros, V.; Thourani, V.; Szeto, W.Y.; et al. Predictors of Mortality and Outcomes of Therapy in Low-Flow Severe Aortic Stenosis. Circulation 2013, 127, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, I.; Maluenda, G.; Iyasu, G.D.; Laynez-Carnicero, A.; Hauville, C.; Torguson, R.; Okubagzi, P.; Xue, Z.; Goldstein, S.A.; Lindsay, J.; et al. Comparison of Outcome of Higher versus Lower Transvalvular Gradients in Patients with Severe Aortic Stenosis and Low (<40%) Left Ventricular Ejection Fraction. Am. J. Cardiol. 2012, 109, 1031–1037. [Google Scholar] [PubMed]
- Lauten, A.; Figulla, H.R.; Möllmann, H.; Holzhey, D.; Kötting, J.; Beckmann, A.; Veit, C.; Cremer, J.; Kuck, K.-H.; Lange, R.; et al. TAVI for low-flow, low-gradient severe aortic stenosis with preserved or reduced ejection fraction: A subgroup analysis from the German Aortic Valve Registry (GARY). EuroIntervention 2014, 10, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.; Rodés-Cabau, J.; Masson, J.; Dumont, E.; De Larochellière, R.; Doyle, D.; Bergeron, S.; Baumgartner, H.; Burwash, I.; Dumesnil, J.; et al. Comparison between Transcatheter and Surgical Prosthetic Valve Implantation in Patients with Severe Aortic Stenosis and Reduced Left Ventricular Ejection Fraction. Circulation 2010, 122, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J. Transcatheter aortic valve implantation: Current and future approaches. Nat. Rev. Cardiol. 2012, 9, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.B.; Lerakis, S.; Gilard, M.; Cavalcante, J.L.; Makkar, R.; Herrmann, H.C.; Windecker, S.; Enriquez-Sarano, M.; Cheema, A.N.; Nombela-Franco, L.; et al. Transcatheter Aortic Valve Replacement in Patients with Low-Flow, Low-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Arnold, S.V.; Herrmann, H.C.; Holmes, D.R.; Szeto, W.Y.; Allen, K.B.; Chhatriwalla, A.K.; Vemulapali, S.; O’brien, S.; Dai, D.; et al. Impact of Ejection Fraction and Aortic Valve Gradient on Outcomes of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2349–2358. [Google Scholar] [CrossRef]
- Connolly, H.M.; Oh, J.K.; Orszulak, T.A.; Osborn, S.L.; Roger, V.L.; Hodge, D.O.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Aortic Valve Replacement for Aortic Stenosis with Severe Left Ventricular Dysfunction. Circulation 1997, 95, 2395–2400. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Berthelot-Richer, M.; Le Ven, F.; Capoulade, R.; Dahou, A.; Dumesnil, J.G.; Mathieu, P.; Pibarot, P. Impact of Classic and Paradoxical Low Flow on Survival after Aortic Valve Replacement for Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2015, 65, 645–653. [Google Scholar] [CrossRef]
- Quere, J.-P.; Monin, J.-L.; Levy, F.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Influence of Preoperative Left Ventricular Contractile Reserve on Postoperative Ejection Fraction in Low-Gradient Aortic Stenosis. Circulation 2006, 113, 1738–1744. [Google Scholar] [CrossRef]
- Monin, J.-L.; Monchi, M.; Kirsch, M.E.; Petit-Eisenmann, H.; Baleynaud, S.; Chauvel, C.; Metz, D.; Adams, C.; Quere, J.-P.; Gueret, P.; et al. Low-gradient aortic stenosis: Impact of prosthesis-patient mismatch on survival. Eur. Heart J. 2007, 28, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, B.; Corbineau, H.; Laurent, M.; Lelong, B.; Langanay, T.; de Place, C.; Froger-Bompas, C.; Leclercq, C.; Daubert, C.; Leguerrier, A. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: Predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005, 91, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Teixeira, R.; Siserman, A.; Puga, L.; Lopes, J.; Sousa, J.P.; Lourenço, C.; Belo, A.; Gonçalves, L. Impact of previous coronary artery bypass grafting in patients presenting with an acute coronary syndrome: Current trends and clinical implications. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Herrmann, S.; Störk, S.; Niemann, M.; Lange, V.; Strotmann, J.M.; Frantz, S.; Beer, M.; Gattenlöhner, S.; Voelker, W.; Ertl, G.; et al. Low-Gradient Aortic Valve Stenosis: Myocardial Fibrosis and Its Influence on Function and Outcome. J. Am. Coll. Cardiol. 2011, 58, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Flores-Umanzor, E.; Nogic, J.; Cepas-Guillén, P.; Hascoet, S.; Pysz, P.; Baz, J.A.; Cruz-González, I.; Amat-Santos, I.J.; Antúnez-Muiños, P.; González, J.C.; et al. Percutaneous paravalvular leak closure after transcatheter aortic valve implantation: The international PLUGinTAVI Registry. EuroIntervention 2023, 19, e442–e449. [Google Scholar] [CrossRef]







| Groups | Mean Transvalvular Gradient | Stroke Volume Index |
|---|---|---|
| Low-flow, high-gradient (LF-HG) | ≥40 mm Hg | ≤35 mL/m2 |
| Low-flow, low-gradient (LF-LG) | <40 mm Hg | ≤35 mL/m2 |
| Normal-flow, high-gradient (NF-HG) | ≥40 mm Hg | >35 mL/m2 |
| Normal-flow, low-gradient (NF-LG) | <40 mm Hg | >35 mL/m2 |
| All Patients (N = 272) | NF-HG AS 95 (34.9%) | LF-HG AS 98 (36%) | NF-LG AS 17 (6.3%) | LF-LG AS 62 (22.8%) | p Value | |
|---|---|---|---|---|---|---|
| Age, yrs | 80 ± 7 | 80 ± 7 | 81 ± 7 | 77 ± 8 | 78 ± 9 | 0.124 |
| BMI, kg/m2 | 26.06 ± 3.17 | 25.32 ± 2.75 | 26.83 ± 3.48 | 26.54 ± 3.64 | 25.67 ± 2.56 | 0.008 |
| Male, (%) | 134 (49.2%) | 34 (35.8%) | 50 (51%) | 9 (52.9%) | 41(65.6%) | 0.018 |
| Diabetes, (%) | 87 (32%) | 32 (33.7%) | 29 (29.6%) | 7 (41.2%) | 19 (31.3%) | 0.623 |
| Hypertension, (%) | 206 (75.7%) | 67 (70.5%) | 76 (77.6%) | 11 (64.7%) | 52 (84.4%) | 0.475 |
| CAD, (%) | 129 (47.4%) | 41 (43.2%) | 44 (44.9%) | 9 (52.9%) | 35 (56.3%) | 0.268 |
| Previous MI, (%) | 55 (20.2%) | 18 (18.9%) | 15 (15.3%) | 3 (17.6%) | 19 (31.3%) | 0.005 |
| Previous PCI, (%) | 36 (13.2%) | 11 (11.2%) | 11 (10.8%) | 2 (14.3%) | 12 (19.2%) | 0.689 |
| Previous CABG, (%) | 63 (23.2%) | 12 (12.4%) | 24 (25.3%) | 6 (35.7%) | 21(34.6%) | 0.025 |
| CKD, (%) | 114 (41.9%) | 36 (37.9%) | 42 (42.9%) | 5 (29.4%) | 31 (50%) | 0.469 |
| PAD, (%) | 100 (36.7%) | 29 (30.5%) | 30 (30.6%) | 4 (23.5%) | 37 (59.4%) | 0.063 |
| COPD, (%) | 71 (26.1%) | 22 (23.2%) | 25 (25.5%) | 7 (41.2%) | 17 (28.1%) | 0.694 |
| EUROSCOREII | 5.14 (4.22–6.48) | 4.50 (3.42–6.0) | 5.15 (4.60–6.48) | 5.62 (4.95–6.88) | 8.34 (4.84–13.58) | 0.004 |
| NYHA III/IV, (%) | 265 (97.4%) | 92 (97.9%) | 96 (98%) | 17 (100)% | 60 (96.9%) | 0.850 |
| All Patients (N = 272) | NFHGAS (N = 95) | LFHGAS (N = 98) | NFLGAS (N = 17) | LFLGAS (N = 62) | p Value | |
|---|---|---|---|---|---|---|
| LVEF, % | 51 ± 9 | 54 ± 6 | 50 ± 9 | 50 ± 11 | 44 ± 12 | <0.001 |
| Mean gradient, mmHg | 50 ± 15 | 57 ± 14 | 53 ± 11 | 34 ± 6 | 32 ± 9 | <0.001 |
| AVA, cm2 | 0.61 ± 0.15 | 0.68 ± 0.14 | 0.52 ± 0.11 | 0.77 ± 0.11 | 0.59 ± 0.16 | <0.001 |
| Moderate-severe MR pre-TAVI | 86 (31.6%) | 19 (20.3%) | 34 (35%) | 7 (38.5%) | 26 (42.9%) | 0.069 |
| Moderate-severe TR pre-TAVI | 64 (23.5%) | 19 (20%) | 28 (28.6%) | 5 (29.4%) | 12 (19.3%) | 0.438 |
| Stroke volume indexed, mL/m2 | 35 ± 11 | 45 ± 9 | 29 ± 6 | 40 ± 4 | 26 ± 5 | <0.001 |
| Pulmonary artery systolic pressure (PASP) (mmHg) | 44.43 ± 12.50 | 44.95 ± 12.52 | 44.98 ± 13.46 | 41.76 ± 8.46 | 42.61 ± 11.22 | 0.620 |
| Severe AV calcification based on MSCT | 172 (63.2%) | 58 (61.4%) | 62 (63.2%) | 14 (80%) | 38 (61.1%) | 0.717 |
| LF-LG AS (N = 62) | NF-LG AS (N = 17) | LF-HG AS (N = 98) | NF-HG AS (N = 95) | p LF-LG AS vs. NF-LG AS | p LF-LG AS vs. LF-HG AS | p LF-LG AS vs. NF-HG AS | p * | |
|---|---|---|---|---|---|---|---|---|
| 1-year all-cause mortality, N (%) | 16 (25.8) | 3 (18.8) | 11 (11.5) | 6 (6.3) | 0.729 | 0.084 | 0.011 | 0.048 |
| 1-year CV mortality, N (%) | 12 (19.3) | 2 (12.5) | 3 (3.1) | 5 (5.7) | 0.701 | 0.008 | 0.067 | 0.018 |
| Stroke at 1-year, N (%) | 2 (3.2%) | 1 (5.9) | 2 (2) | 2 (2.1) | 1.000 | 1.000 | 0.802 | 0.868 |
| 1-year MACCE (death, non-fatal MI, non-fatal stroke), N (%) | 16 (25.8) | 3 (18.8) | 12 (12.2) | 8 (8.4) | 1.000 | 0.092 | 0.029 | 0.097 |
| 5-year all-cause mortality, N (%) | 40 (64.5) | 10 (58.8) | 47 (47.9) | 40 (42.5) | 1.000 | 0.152 | 0.038 | 0.047 |
| 5-year CV mortality, N (%) | 33 (53.2) | 4 (23.5) | 21 (21.4) | 17 (17.9) | 0.075 | 0.001 | 0.001 | 0.002 |
| LF-LG (N = 62) | NF-LG (N = 17) | LF-HG (N = 98) | NF-HG (N = 95) | p LF-LG vs. NF-LG | p LF-LG vs. LF-HG | p LF-LG vs. NF-HG | p * | |
|---|---|---|---|---|---|---|---|---|
| 30-day PPI N (%) | 31 (50) | 7 (41.2) | 41(41.8) | 34 (35.8) | 0.683 | 0.419 | 0.147 | 0.561 |
| 30-day Major vascular complications N (%) | 2 (3.2) | 1 (5.9) | 8 (8.2) | 7 (7.4) | 0.660 | 0.294 | 0.353 | 0.755 |
| 30-day Life-threatening/major bleeding complications N (%) | 9 (14.5) | 2 (11.7) | 12 (12.2) | 15(15.8) | 0.659 | 0.622 | 0.965 | 0.863 |
| 95.0% CI for Exp(B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper | |
| EUROSCORE II | −0.025 | 0.016 | 2.455 | 1 | 0.117 | 0.975 | 0.945 | 1.006 |
| CHRONIC KIDNEY DISEASE | 0.429 | 0.275 | 2.441 | 1 | 0.118 | 1.536 | 0.896 | 2.633 |
| PRIOR MI | 0.139 | 0.301 | 0.213 | 1 | 0.645 | 1.149 | 0.637 | 2.072 |
| Pre-TAVI MR ≥ moderate | 0.069 | 0.291 | 0.057 | 1 | 0.812 | 1.072 | 0.606 | 1.894 |
| Pre-TAVI TR ≥ moderate | 1.128 | 0.322 | 12.288 | 1 | 0.000 | 3.091 | 1.645 | 5.809 |
| Pre-TAVI PASP | 0.005 | 0.012 | 0.194 | 1 | 0.660 | 1.005 | 0.983 | 1.028 |
| Pre-TAVI SVi | −0.051 | 0.018 | 8.316 | 1 | 0.004 | 0.951 | 0.918 | 0.984 |
| Post-TAVI PASP | 0.010 | 0.013 | 0.641 | 1 | 0.423 | 1.010 | 0.985 | 1.036 |
| Post-TAVI PVL ≥ moderate | 1.105 | 0.288 | 11.677 | 1 | 0.042 | 1.456 | 1.106 | 1.792 |
| FLOW-GRADIENT STATE * US * | 9.030 | 3 | 0.029 | |||||
| LF-HG AS | −0.374 | 0.505 | 0.548 | 1 | 0.459 | 0.688 | 0.256 | 1.852 |
| NF-LG AS | 0.564 | 0.553 | 1.039 | 1 | 0.308 | 0.360 | 0.594 | 5.197 |
| LF-LG AS | −1.022 | 0.433 | 5.572 | 1 | 0.018 | 1.757 | 0.154 | 0.841 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Standardized Coefficient (Beta) | R2 | p Value | Standardized Coefficient (Beta) | R2 | p Value | |
| Clinical variables | ||||||
| Age, yrs | −0.021 | 0.0005 | 0.856 | |||
| Male | 0.156 | 0.021 | 0.265 | |||
| BMI, kg/m2 | 0.102 | 0.014 | 0.288 | |||
| Hypertension | −0.078 | 0.006 | 0.724 | |||
| Diabetes | −0.092 | 0.008 | 0.623 | |||
| NYHA functional class III–IV | −0.062 | 0.006 | 0.621 | |||
| CAD | −0.188 | 0.032 | 0.088 | |||
| Previous MI | −0.155 | 0.024 | 0.045 | −0.193 | 0.036 | 0.054 |
| Previous PCI | −0.204 | 0.053 | 0.065 | |||
| Previous CABG | −0.242 | 0.068 | 0.052 | |||
| CKD | 0.102 | 0.009 | 0.355 | |||
| PAD | 0.126 | 0.012 | 0.248 | |||
| COPD | −0.036 | 0.0008 | 0.726 | |||
| EUROSCORE II | 0.074 | 0.009 | 0.273 | |||
| Baseline echocardiographic variables | ||||||
| LVEF, % | −0.922 | 0.261 | <0.001 | −0.906 | 0.274 | <0.001 |
| Mean gradient, mmHg | ||||||
| AVA, cm2 | ||||||
| Moderate-severe MR | ||||||
| Moderate-severe TR | ||||||
| Stroke Volume indexed, mL/m2 | −0.875 | 0.211 | <0.001 | −0.898 | 0.211 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikonomou, G.; Apostolos, A.; Drakopoulou, M.; Simopoulou, C.; Karmpalioti, M.; Toskas, P.; Stathogiannis, K.; Xanthopoulou, M.; Ktenopoulos, N.; Latsios, G.; et al. Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 1200. https://doi.org/10.3390/jcm13051200
Oikonomou G, Apostolos A, Drakopoulou M, Simopoulou C, Karmpalioti M, Toskas P, Stathogiannis K, Xanthopoulou M, Ktenopoulos N, Latsios G, et al. Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2024; 13(5):1200. https://doi.org/10.3390/jcm13051200
Chicago/Turabian StyleOikonomou, George, Anastasios Apostolos, Maria Drakopoulou, Chryssavgi Simopoulou, Maria Karmpalioti, Pantelis Toskas, Konstantinos Stathogiannis, Maria Xanthopoulou, Nikolaos Ktenopoulos, George Latsios, and et al. 2024. "Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 13, no. 5: 1200. https://doi.org/10.3390/jcm13051200