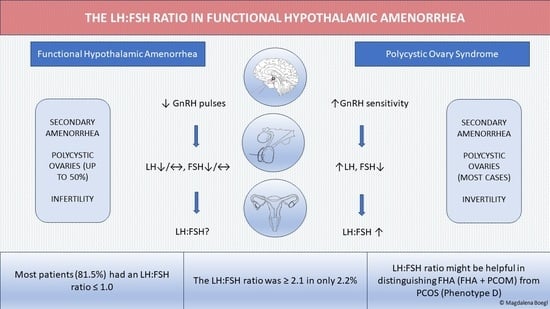
The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, D.A.; Paradise, S.L.; Reeder, R.M. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am. Fam. Physician 2019, 100, 39–48. [Google Scholar]
- Munster, K.; Helm, P.; Schmidt, L. Secondary amenorrhoea: Prevalence and medical contact--a cross-sectional study from a Danish county. Br. J. Obstet. Gynaecol. 1992, 99, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M. Clinical practice. Functional hypothalamic amenorrhea. N. Engl. J. Med. 2010, 363, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.; Dewailly, D.; Marculescu, R.; Ghobrial, S.; Parry, J.P.; Ott, J. Stress and polycystic ovarian morphology in functional hypothalamic amenorrhea: A retrospective cohort study. Reprod. Biol. Endocrinol. 2023, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, F.; Politi, G.; Leone, D.; Vegni, E.; Borghi, L. Psychological factors in functional hypothalamic amenorrhea: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 981491. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Torbati, T.; Dutra, E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Blenck, C.L.; Harvey, P.A.; Reckelhoff, J.F.; Leinwand, L.A. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ. Res. 2016, 118, 1294–1312. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Pedrielli, G.; Bosoni, D.; Tiranini, L.; Cucinella, L.; Calogero, A.E.; Facchinetti, F.; Nappi, R.E. Sexual functioning in women with functional hypothalamic amenorrhea: Exploring the relevance of an underlying polycystic ovary syndrome (PCOS)-phenotype. J. Endocrinol. Investig. 2023, 46, 1623–1632. [Google Scholar] [CrossRef]
- Fontana, L.; Garzia, E.; Marfia, G.; Galiano, V.; Miozzo, M. Epigenetics of functional hypothalamic amenorrhea. Front. Endocrinol. 2022, 13, 953431. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Phylactou, M.; Clarke, S.A.; Patel, B.; Baggaley, C.; Jayasena, C.N.; Kelsey, T.W.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2021, 95, 239–252. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of the International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2023, 120, 767–793. [Google Scholar] [CrossRef]
- Makolle, S.; Catteau-Jonard, S.; Robin, G.; Dewailly, D. Revisiting the serum level of anti-Mullerian hormone in patients with functional hypothalamic anovulation. Hum. Reprod. 2021, 36, 1043–1051. [Google Scholar] [CrossRef]
- Beitl, K.; Dewailly, D.; Seemann, R.; Hager, M.; Bunker, J.; Mayrhofer, D.; Holzer, I.; Ott, J. Polycystic Ovary Syndrome Phenotype D versus Functional Hypothalamic Amenorrhea with Polycystic Ovarian Morphology: A Retrospective Study about a Frequent Differential Diagnosis. Front. Endocrinol. 2022, 13, 904706. [Google Scholar] [CrossRef] [PubMed]
- Piltonen, T.T.; Komsi, E.; Morin-Papunen, L.C.; Korhonen, E.; Franks, S.; Järvelin, M.-R.; Arffman, R.K.; Ollila, M.-M. AMH as part of the diagnostic PCOS workup in large epidemiological studies. Eur. J. Endocrinol. 2023, 188, 547–554. [Google Scholar] [CrossRef]
- Berga, S.L.; Mortola, J.F.; Girton, L.; Suh, B.; Laughlin, G.; Pham, P.; Yen, S.S. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1989, 68, 301–308. [Google Scholar] [CrossRef]
- Morrison, A.E.; Fleming, S.; Levy, M.J. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin. Endocrinol. 2021, 95, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jonard, S.; Pigny, P.; Jacquesson, L.; Demerle-Roux, C.; Robert, Y.; Dewailly, D. The ovarian markers of the FSH insufficiency in functional hypothalamic amenorrhoea. Hum. Reprod. 2005, 20, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Meczekalski, B.; Podfigurna-Stopa, A.; Santagni, S.; Rattighieri, E.; Ricchieri, F.; Chierchia, E.; Simoncini, T. Estriol administration modulates luteinizing hormone secretion in women with functional hypothalamic amenorrhea. Fertil. Steril. 2012, 97, 483–488. [Google Scholar] [CrossRef]
- Marshall, J.C.; Dalkin, A.C.; Haisenleder, D.J.; Griffin, M.L.; Kelch, R.P. GnRH pulses—The regulators of human reproduction. Trans. Am. Clin. Climatol. Assoc. 1993, 104, 31–46. [Google Scholar]
- Tsutsumi, R.; Webster, N.J. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 2009, 56, 729–737. [Google Scholar] [CrossRef]
- Herpertz, S.; Hagenah, U.; Vocks, S.; von Wietersheim, J.; Cuntz, U.; Zeeck, A. The diagnosis and treatment of eating disorders. Dtsch. Arztebl. Int. 2011, 108, 678–685. [Google Scholar] [CrossRef]
- Schneider, L.F.; Warren, M.P. Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil. Steril. 2006, 86, 1744–1749. [Google Scholar] [CrossRef]
- Tinahones, F.J.; Martinez-Alfaro, B.; Gonzalo-Marin, M.; Garcia-Almeida, J.M.; Garrido-Sanchez, L.; Cardona, F. Recovery of menstrual cycle after therapy for anorexia nervosa. Eat. Weight. Disord. 2005, 10, e52–e55. [Google Scholar] [CrossRef]
- Hager, M.; Ott, J.; Marschalek, J.; Marschalek, M.L.; Kinsky, C.; Marculescu, R.; Dewailly, D. Basal and dynamic relationships between serum anti-Mullerian hormone and gonadotropins in patients with functional hypothalamic amenorrhea, with or without polycystic ovarian morphology. Reprod. Biol. Endocrinol. 2022, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, D.; Dewailly, D.; Hager, M.; Marculescu, R.; Beitl, K.; Ott, J. Functional hypothalamic amenorrhea with or without polycystic ovarian morphology: A retrospective cohort study about insulin resistance. Fertil. Steril. 2022, 118, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Update 2014, 20, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Robin, G.; Gallo, C.; Catteau-Jonard, S.; Lefebvre-Maunoury, C.; Pigny, P.; Duhamel, A.; Dewailly, D. Polycystic Ovary-Like Abnormalities (PCO-L) in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 2012, 97, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Fruzzetti, F.; Lobo, R.A. Features of polycystic ovary syndrome (PCOS) in women with functional hypothalamic amenorrhea (FHA) may be reversible with recovery of menstrual function. Gynecol. Endocrinol. 2018, 34, 301–304. [Google Scholar] [CrossRef]
- Alvero, R.; Kimzey, L.; Sebring, N.; Reynolds, J.; Loughran, M.; Nieman, L.; Olson, B.R. Effects of fasting on neuroendocrine function and follicle development in lean women. J. Clin. Endocrinol. Metab. 1998, 83, 76–80. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 77 (Suppl. S4), S3–S5. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Belda, X.; Fuentes, S.; Daviu, N.; Nadal, R.; Armario, A. Stress-induced sensitization: The hypothalamic-pituitary-adrenal axis and beyond. Stress 2015, 18, 269–279. [Google Scholar] [CrossRef]
- Selzer, C.; Ott, J.; Dewailly, D.; Marculescu, R.; Steininger, J.; Hager, M. Prolactin levels in Functional hypothalamic amenorrhea: A retrospective case-control study. Arch. Gynecol. Obstet. 2023, 309, 651–658. [Google Scholar] [CrossRef]
- Macotela, Y.; Triebel, J.; Clapp, C. Time for a New Perspective on Prolactin in Metabolism. Trends Endocrinol. Metab. 2020, 31, 276–286. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.; Endocrine, S. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, J.; Blizzard, L.; Oddy, W.H.; Dwyer, T.; Bazzano, L.A.; Hickey, M.; Harville, E.W.; Venn, A.J. Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: The Childhood Determinants of Adult Health Study and the Bogalusa Heart Study. Hum. Reprod. 2020, 35, 1185–1198. [Google Scholar] [CrossRef]
- Ezeh, U.; Pisarska, M.D.; Azziz, R. Association of severity of menstrual dysfunction with hyperinsulinemia and dysglycemia in polycystic ovary syndrome. Hum. Reprod. 2022, 37, 553–564. [Google Scholar] [CrossRef]
- La Marca, A.; Pati, M.; Orvieto, R.; Stabile, G.; Carducci Artenisio, A.; Volpe, A. Serum anti-mullerian hormone levels in women with secondary amenorrhea. Fertil. Steril. 2006, 85, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lie Fong, S.; Schipper, I.; Valkenburg, O.; de Jong, F.H.; Visser, J.A.; Laven, J.S. The role of anti-Mullerian hormone in the classification of anovulatory infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 186, 75–79. [Google Scholar] [CrossRef]
- Luisi, S.; Ciani, V.; Podfigurna-Stopa, A.; Lazzeri, L.; De Pascalis, F.; Meczekalski, B.; Petraglia, F. Serum anti-Mullerian hormone, inhibin B, and total inhibin levels in women with hypothalamic amenorrhea and anorexia nervosa. Gynecol. Endocrinol. 2012, 28, 34–38. [Google Scholar] [CrossRef]
- Qu, X.; Donnelly, R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2020, 21, 8191. [Google Scholar] [CrossRef]


| Age (years), median (IQR) 1 | 26 (22;29) |
| BMI (kg/m2), median (IQR) 1 | 20.3 (18.6;22.0) |
| Gravidity: n (%) 2 | |
| 0 | 134 (99.3) |
| 1 | 1 (0.7) |
| Parity: n (%) 2 | |
| 0 | 134 (99.3) |
| 1 | 1 (0.7) |
| Causes for FHA: n (%) 2,3 | |
| Stress | 44 (32.6) |
| Excessive exercise | 55 (40.7) |
| Anorexia nervosa | 30 (22.2) |
| Acute weight loss | 33 (24.4) |
| Underweight | 24 (17.8) |
| Duration since last menstrual bleeding (months), median (IQR) 1 | 14 (10;24) |
| Hormones, median (IQR) 1 | |
| TSH (IU/mL) | 1.57 (1.12;2.03) |
| Prolactin (ng/mL) | 8.9 (6.6;12.9) |
| FSH (mIU/mL) | 4.7 (3.3;6.5) |
| LH (mIU/mL) | 2.6 (1.3;4.7) |
| Estradiol (pg/mL) | 23 (12;31) |
| Testosterone (ng/mL) | 0.20 (0.13;0.29) |
| DHEAS (µg/mL) | 2.03 (1.40;2.73) |
| SHBG (nmol/L) | 73.0 (55.1;101.8) |
| AMH (ng/mL) | 3.1 (1.6;6.2) |
| Polycystic ovarian morphology on ultrasound, n (%) 2 | 58 (43.0) |
| FSH | LH | LH:FSH Ratio | Estradiol | Testosterone | AMH | Prolactin | ||
|---|---|---|---|---|---|---|---|---|
| FSH | r2 | - | 0.556 | −0.141 | 0.291 | 0.045 | 0.221 | 0.323 |
| p | <0.001 | 0.103 | <0.001 | 0.604 | 0.010 | 0.134 | ||
| LH | r2 | 0.556 | - | 0.633 | 0.387 | 0.218 | 0.060 | 0.315 |
| p | <0.001 | <0.001 | <0.001 | 0.011 | 0.134 | <0.001 | ||
| LH:FSH ratio | r2 | −0.141 | 0.633 | - | 0.271 | 0.159 | −0.076 | 0.261 |
| p | 0.103 | <0.001 | 0.002 | 0.066 | 0.385 | 0.002 | ||
| Estradiol | r2 | 0.291 | 0.387 | 0.271 | - | 0.243 | 0.076 | 0.182 |
| p | <0.001 | <0.001 | 0.002 | 0.005 | 0.386 | 0.037 | ||
| Testosterone | r2 | 0.045 | 0.218 | 0.159 | 0.243 | - | 0.126 | 0.268 |
| p | 0.604 | 0.011 | 0.066 | 0.005 | 0.134 | 0.002 | ||
| AMH | r2 | 0.221 | 0.060 | −0.076 | 0.076 | 0.126 | - | −0.051 |
| p | 0.010 | 0.134 | 0.385 | 0.386 | 0.134 | 0.555 | ||
| Prolactin | r2 | 0.323 | 0.315 | 0.261 | 0.182 | 0.268 | −0.051 | - |
| p | 0.134 | <0.001 | 0.002 | 0.037 | 0.002 | 0.555 |
| LH:FSH Ratio ≤ 1 (n = 110) | LH:FSH Ratio > 1 (n = 25) | OR (95%CI) | p | OR (95%CI) | p | |
|---|---|---|---|---|---|---|
| Age (years) 1 | 26 (22;29) | 24 (22;28) | 1.047 (0.956;1.147) | 0.321 | - | - |
| BMI (kg/m2) 1 | 19.9 (18.6;21.7) | 21.2 (18.6;22.3) | 0.880 (0.740;1.047) | 0.149 | - | - |
| Causes for FHA: | ||||||
| Stress 2,3 | 33 (30.0) | 11 (44.0) | 0.545 (0.224;1.327) | 0.181 | - | - |
| Excessive exercise 2,3 | 45 (40.9) | 10 (40.0) | 1.038 (0.428;2.518) | 0.933 | - | - |
| Anorexia nervosa 2,3 | 22 (20.0) | 8 (32.0) | 0.531 (0.203;1.389) | 0.197 | - | - |
| Acute weight loss 2,3 | 27 (24.5) | 6 (24.0) | 1.030 (0.373;2.844) | 0.954 | - | - |
| Underweight 2,3 | 19 (17.3) | 5 (20.0) | 0.835 (0.279;2.503) | 0.748 | - | - |
| Duration since last bleeding (months) 1 | 14 (12;24) | 12 (8;16) | 1.039 (0.998;1.082) | 0.065 | - | - |
| FSH (mIU/mL) 1 | 4.7 (3.4;6.3) | 4.7 (2.6;6.5) | 1.056 (0.868;1.284) | 0.588 | - | - |
| LH (mIU/mL) 1 | 2.2 (1.2;3.5) | 6.3 (4.0;7.7) | 0.499 (0.385;0.647) | <0.001 | 0.520 (0.400;0.675) | <0.001 |
| Prolactin (ng/mL) 1 | 8.1 (6.4;12.4) | 12.0 (8.8;14.2) | 0.960 (0.894;1.031) | 0.260 | - | - |
| Estradiol (pg/mL) 1 | 21 (11;28) | 25 (22;39) | 0.956 (0.928;0.986) | 0.004 | 0.975 (0.939;1.013) | 0.196 |
| Testosterone (ng/mL) 1 | 0.19 (0.13;0.29) | 0.25 (0.14;0.33) | 0.015 (0.000;1.030) | 0.052 | - | - |
| SHBG (nmol/L) 1 | 74.0 (57.4;99.1) | 70.0 (48.6;113.0) | 0.998 (0.986;1.010) | 0.720 | - | - |
| AMH (ng/mL) 1 | 2.8 (1.6;6.1) | 4.5 (2.2;6.2) | 0.955 (0.863;1.087) | 0.486 | - | - |
| PCOM 2 | 42 (38.2) | 13 (52.0) | 0.570 (0.238;1.366) | 0.208 | - | - |
| PCOM (n = 58) | Non-PCOM (n = 77) | p | |
|---|---|---|---|
| Age (years) | 26 (22;28) | 26 (22;30) | 0.299 |
| BMI (kg/m2) | 20.4 (18.8;22.5) | 20.0 (18.4;21.4) | 0.190 |
| Prolactin (ng/mL) | 9.6 (6.6;13.0) | 8.8 (6.7;12.8) | 0.964 |
| FSH (mIU/mL) | 4.8 (3.5;6.5) | 4.7 (3.2;6.2) | 0.426 |
| LH (mIU/mL) | 2.8 (1.5;5.5) | 2.6 (1.3;4.6) | 0.290 |
| LH:FSH ratio | 0.8 (0.3;1.0) | 0.7 (0.4;1.0) | 0.728 |
| Estradiol (pg/mL) | 24 (11;34) | 22 (12;29) | 0.719 |
| Testosterone (ng/mL) | 0.20 (0.14;0.29) | 0.19 (0.13;0.29) | 0.881 |
| SHBG (nmol/L) | 67.0 (39.7;94.1) | 79.4 (63.3;104.0) | 0.008 |
| AMH (ng/mL) | 6.3 (4.9;7.6) | 2.0 (1.1;2.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boegl, M.; Dewailly, D.; Marculescu, R.; Steininger, J.; Ott, J.; Hager, M. The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study. J. Clin. Med. 2024, 13, 1201. https://doi.org/10.3390/jcm13051201
Boegl M, Dewailly D, Marculescu R, Steininger J, Ott J, Hager M. The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study. Journal of Clinical Medicine. 2024; 13(5):1201. https://doi.org/10.3390/jcm13051201
Chicago/Turabian StyleBoegl, Magdalena, Didier Dewailly, Rodrig Marculescu, Johanna Steininger, Johannes Ott, and Marlene Hager. 2024. "The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study" Journal of Clinical Medicine 13, no. 5: 1201. https://doi.org/10.3390/jcm13051201