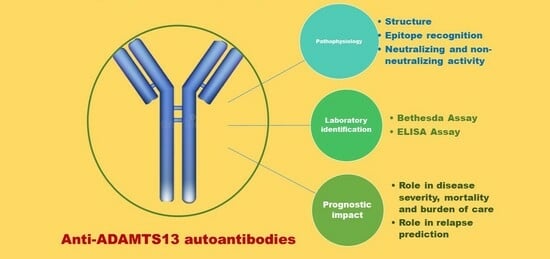
Anti-ADAMTS13 Autoantibodies: From Pathophysiology to Prognostic Impact—A Review for Clinicians
Abstract
:1. Introduction
2. Anti-ADAMTS13 Autoantibodies Pathophysiology: Production, Structure and Function
3. Anti-ADAMTS13 Autoantibodies Detection
4. Anti-ADAMTS13 Autoantibodies and Prognostic Role
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.L. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu. Rev. Med. 2015, 66, 211–225. [Google Scholar] [PubMed]
- Shin, J.S.; Subhan, M.O.; Cambridge, G.; Guo, Y. Alterations in B- and circulating T-follicular helper cell subsets in immune thrombotic thrombocytopenic purpura. Blood Adv. 2022, 6, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Sorvillo, N.; van Haren, S.D.; Kaijen, P.H.; ten Brinke, A. Preferential HLA-DRB1*11-dependent presentation of CUB2-derived peptides by ADAMTS13-pulsed dendritic cells. Blood 2013, 121, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Verbij, F.C.; Turksma, A.W.; de Heij, F.; Kaijen, P. CD4+ T cells from patients with acquired thrombotic thrombocytopenic purpura recognize CUB2 domain-derived peptides. Blood 2016, 127, 1606–1609. [Google Scholar] [PubMed]
- Carsetti, R.; Rosado, M.M.; Wardmann, H. Peripheral development of B cells in mouse and man. Immunol. Rev. 2004, 197, 179–191. [Google Scholar] [CrossRef]
- Sitaru, C.; Mihai, S.; Zillikens, D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch. Dermatol. Res. 2007, 299, 1–8. [Google Scholar]
- Maran, R.; Dueymes, M.; Le Corre, R.; Renaudineau, Y.; Shoenfeld, Y. IgG subclasses of human autoantibodies. Ann. Med. Interne 1997, 148, 29–38. [Google Scholar]
- Ferrari, S.; Mudde, G.C.; Rieger, M.; Veyradier, A. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2009, 7, 1703–1710. [Google Scholar] [CrossRef]
- Zheng, X.; Chung, D.; Takayama, T.K.; Majerus, E.M. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J. Biol. Chem. 2001, 276, 41059–41063. [Google Scholar] [CrossRef]
- South, K.; Luken, B.M.; Crawley, J.T.; Phillips, R. Conformational activation of ADAMTS13. Proc. Natl. Acad. Sci. USA 2014, 111, 18578–18583. [Google Scholar] [CrossRef]
- Roose, E.; Schelpe, A.S.; Tellier, E.; Sinkovits, G. Open ADAMTS13, induced by antibodies, is a biomarker for subclinical immune-mediated thrombotic thrombocytopenic purpura. Blood 2020, 136, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Roose, E.; Vidarsson, G.; Kangro, K.; Verhagen, O.J.H.M. Anti-ADAMTS13 Autoantibodies against Cryptic Epitopes in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Thromb. Haemost. 2018, 118, 1729–1742. [Google Scholar] [PubMed]
- De Waele, L.; Curie, A.; Kangro, K.; Tellier, E. Anti-cysteine/spacer antibodies that open ADAMTS13 are a common feature in iTTP. Blood Adv. 2021, 5, 4480–4484. [Google Scholar] [CrossRef] [PubMed]
- Luken, B.M.; Turenhout, E.A.; Hulstein, J.J.; Van Mourik, J.A. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb. Haemost. 2005, 93, 267–274. [Google Scholar] [CrossRef]
- Luken, B.M.; Kaijen, P.H.; Turenhout, E.A.; Kremer Hovinga, J.A. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2006, 4, 2355–2364. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Moriki, T.; Igari, A.; Nakagawa, T. Epitope analysis of autoantibodies to ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Thromb. Res. 2011, 128, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Velásquez Pereira, L.C.; Roose, E.; Graça, N.A.G.; Sinkovits, G. Immunogenic hotspots in the spacer domain of ADAMTS13 in immune-mediated thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2021, 19, 478–488. [Google Scholar] [CrossRef]
- Klaus, C.; Plaimauer, B.; Studt, J.D.; Dorner, F. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood 2004, 103, 4514–4519. [Google Scholar] [CrossRef]
- Ostertag, E.M.; Kacir, S.; Thiboutot, M.; Gulendran, G. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 1. Structural and functional characterization in vitro. Transfusion 2016, 56, 1763–1774. [Google Scholar] [CrossRef]
- Thomas, M.R.; de Groot, R.; Scully, M.A.; Crawley, J.T. Pathogenicity of Anti-ADAMTS13 Autoantibodies in Acquired Thrombotic Thrombocytopenic Purpura. EBioMedicine 2015, 2, 942–952. [Google Scholar]
- Kangro, K.; Roose, E.; Joly, B.S.; Sinkovits, G. Anti-ADAMTS13 autoantibody profiling in patients with immune-mediated thrombotic thrombocytopenic purpura. Blood Adv. 2021, 5, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Matsumoto, M.; De Waele, L.; Dekimpe, C. ADAMTS13 conformation and immunoprofiles in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura. Blood Adv. 2023, 7, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Graça, N.A.G.; Ercig, B.; Carolina Velasquez Pereira, L.; Kangro, K. Modifying ADAMTS13 to modulate binding of pathogenic autoantibodies of patients with acquired thrombotic thrombocytopenic purpura. Haematologica 2020, 105, 2619–2630. [Google Scholar] [CrossRef]
- Jian, C.; Xiao, J.; Gong, L.; Skipwith, C.G.; Jin, S.Y. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood 2012, 119, 3836–3843. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Matsumoto, M.; Kokame, K.; Yagi, H. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood 2003, 102, 3232–3237. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Knöbl, P.; Kolovratova, V.; Plaimauer, B. Inverse correlation of free and immune complex-sequestered anti-ADAMTS13 antibodies in a patient with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2012, 10, 156–158. [Google Scholar] [CrossRef]
- Ferrari, S.; Palavra, K.; Gruber, B.; Kremer Hovinga, J.A.; Knöbl, P.; Caron, C.; Cromwell, C.; Aledort, L.; Plaimauer, B.; Turecek, P.L.; et al. Persistence of circulating ADAMTS13-specific immune complexes in patients with acquired thrombotic thrombocytopenic purpura. Haematologica 2014, 99, 779–787. [Google Scholar] [CrossRef]
- Lotta, L.A.; Valsecchi, C.; Pontiggia, S.; Mancini, I. Measurement and prevalence of circulating ADAMTS13-specific immune complexes in autoimmune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2014, 12, 329–336. [Google Scholar] [CrossRef]
- Vugmeyster, Y.; Xu, X.; Theil, F.P.; Khawli, L.A. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J. Biol. Chem. 2012, 3, 73–92. [Google Scholar] [CrossRef]
- Schifferli, J.A.; Taylor, R.P. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989, 35, 993–1003. [Google Scholar] [CrossRef]
- Emlen, W.; Carl, V.; Burdick, G. Mechanism of transfer of immune complexes from red blood cell CR1 to monocytes. Clin. Exp. Immunol. 1992, 89, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Erlandsson, A.; Eriksson, D.; Ullén, A. Idiotypic-anti-idiotypic complexes and their in vivo metabolism. Cancer 2002, 94 (Suppl. S4), 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, I.; Muro, H.; Shirasawa, H.; Ito, I. Endocytosis of soluble IgG immune complex and its transport to lysosomes in hepatic sinusoidal endothelial cells. J. Hepatol. 1992, 16, 106–114. [Google Scholar] [CrossRef]
- Underwood, M.I.; Alwan, F.; Thomas, M.R.; Scully, M.A. Autoantibodies enhance ADAMTS-13 clearance in patients with immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2023, 21, 1544–1552. [Google Scholar] [CrossRef]
- Smock, K.J. ADAMTS13 testing update: Focus on laboratory aspects of difficult thrombotic thrombocytopenic purpura diagnoses and effects of new therapies. Int. J. Lab. Hematol. 2021, 43 (Suppl. S1), 103–108. [Google Scholar] [CrossRef]
- Page, E.E.; Kremer Hovinga, J.A.; Terrell, D.R.; Vesely, S.K. Thrombotic thrombocytopenic purpura: Diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017, 1, 590–600. [Google Scholar] [CrossRef]
- Hubbard, A.R.; Heath, A.B.; Kremer Hovinga, J.A. Subcommittee on von Willebrand Factor. Establishment of the WHO 1st International Standard ADAMTS13, plasma (12/252): Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 1151–1153. [Google Scholar] [CrossRef]
- George, J.N. The remarkable diversity of thrombotic thrombocytopenic purpura: A perspective. Blood Adv. 2018, 2, 1510–1516. [Google Scholar] [CrossRef]
- Peyvandi, F.; Palla, R.; Lotta, L.A.; Mackie, I. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2010, 8, 631–640. [Google Scholar] [CrossRef]
- Moore, G.W.; Vetr, H.; Binder, N.B. ADAMTS13 Antibody and Inhibitor Assays. Methods Mol. Biol. 2023, 2663, 549–565. [Google Scholar]
- Vendramin, C.; Thomas, M.; Westwood, J.P.; Scully, M. Bethesda Assay for Detecting Inhibitory Anti-ADAMTS13 Antibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura. TH Open 2018, 2, e329–e333. [Google Scholar] [CrossRef]
- Kremer Hovinga, J.A.; Heeb, S.R.; Skowronska, M.; Schaller, M. Pathophysiology of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. J. Thromb. Haemost. 2018, 16, 618–629. [Google Scholar] [CrossRef]
- Rieger, M.; Mannucci, P.M.; Kremer Hovinga, J.A.; Herzog, A. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Shelat, S.G.; Ai, J.; Zheng, X.L. Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays. Semin. Thromb. Hemost. 2005, 31, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Dekimpe, C.; Roose, E.; Kangro, K.; Bonnez, Q. Determination of anti-ADAMTS-13 autoantibody titers in ELISA: Influence of ADAMTS-13 presentation and autoantibody detection. J. Thromb. Haemost. 2021, 19, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Masias, C.; Cataland, S.R. The role of ADAMTS13 testing in the diagnosis and management of thrombotic microangiopathies and thrombosis. Blood 2018, 132, 903–910. [Google Scholar]
- Bettoni, G.; Palla, R.; Valsecchi, C.; Consonni, D. ADAMTS-13 activity and autoantibodies classes and subclasses as prognostic predictors in acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2012, 10, 1556–1565. [Google Scholar] [CrossRef]
- Roos, A.; Bouwman, L.H.; van Gijlswijk-Janssen, D.J.; Faber-Krol, M.C. Human IgA activates the complement system via the mannan-binding lectin pathway. J. Immunol. 2001, 167, 2861–2868. [Google Scholar] [CrossRef]
- Zheng, X.L.; Kaufman, R.M.; Goodnough, L.T.; Sadler, J.E. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood 2004, 103, 4043–4049. [Google Scholar] [CrossRef]
- Vesely, S.K.; George, J.N.; Lämmle, B.; Studt, J.D. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: Relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 2003, 102, 60–68. [Google Scholar] [CrossRef]
- Veyradier, A.; Obert, B.; Houllier, A.; Meyer, D. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: A study of 111 cases. Blood 2001, 98, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Alwan, F.; Vendramin, C.; Vanhoorelbeke, K.; Langley, K. Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood 2017, 130, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Wolf, M.; Veyradier, A.; Bussel, A. Réseau d’Etude des Microangiopathies Thrombotiques de l’Adulte. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br. J. Haematol. 2006, 132, 66–74. [Google Scholar] [CrossRef]
- Prasannan, N.; Thomas, M.; Stubbs, M.; Westwood, J.P. Delayed normalization of ADAMTS13 activity in acute thrombotic thrombocytopenic purpura in the caplacizumab era. Blood 2023, 141, 2206–2213. [Google Scholar] [PubMed]
- Dainese, C.; Valeri, F.; Pizzo, E.; Valpreda, A. ADAMTS13 Autoantibodies and Burden of Care in Immune Thrombotic Thrombocytopenic purpura: New Evidence and Future Implications. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221125785. [Google Scholar] [CrossRef] [PubMed]
- Jestin, M.; Benhamou, Y.; Schelpe, A.S.; Roose, E. French Thrombotic Microangiopathies Reference Center. Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura. Blood 2018, 132, 2143–2153. [Google Scholar]
- Mai Falk, J.; Scharrer, I. Idiopathic thrombotic thrombocytopenic purpura: Strongest risk factor for relapse from remission is having had a relapse. Transfusion 2016, 56, 2819–2823. [Google Scholar]
- Liu, A.; Mazepa, M.; Davis, E.; Johnson, A.; Antun, A.G.; Farland, A.M.; Woods, R.R.; Metjian, A.; Bagby, K.; Park, Y. African American race is associated with decreased relapse-free survival in immune thrombotic thrombocytopenic purpura. Blood 2019, 134 (Suppl. S1), 1066. [Google Scholar]
- Peyvandi, F.; Lavoretano, S.; Palla, R.; Feys, H.B. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica 2008, 93, 232–239. [Google Scholar] [CrossRef]
- Schieppati, F.; Russo, L.; Marchetti, M.; Barcella, L. Low levels of ADAMTS-13 with high anti-ADAMTS-13 antibodies during remission of immune-mediated thrombotic thrombocytopenic purpura highly predict for disease relapse: A multi-institutional study. Am. J. Hematol. 2020, 95, 953–959. [Google Scholar] [CrossRef]
- Jin, M.; Casper, T.C.; Cataland, S.R.; Kennedy, M.S. Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br. J. Haematol. 2008, 141, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.; Ferrari, B.; Valsecchi, C.; Pontiggia, S. Italian Group of TTP Investigators. ADAMTS13-specific circulating immune complexes as potential predictors of relapse in patients with acquired thrombotic thrombocytopenic purpura. Eur. J. Intern. Med. 2017, 39, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.; Vernant, J.P.; Veyradier, A.; Wolf, M. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: A study of 11 cases. Blood 2005, 106, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Bresin, E.; Gastoldi, S.; Daina, E.; Belotti, D. Rituximab as pre-emptive treatment in patients with thrombotic thrombocytopenic purpura and evidence of anti-ADAMTS13 autoantibodies. Thromb. Haemost. 2009, 101, 233–238. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dainese, C.; Valeri, F.; Bruno, B.; Borchiellini, A. Anti-ADAMTS13 Autoantibodies: From Pathophysiology to Prognostic Impact—A Review for Clinicians. J. Clin. Med. 2023, 12, 5630. https://doi.org/10.3390/jcm12175630
Dainese C, Valeri F, Bruno B, Borchiellini A. Anti-ADAMTS13 Autoantibodies: From Pathophysiology to Prognostic Impact—A Review for Clinicians. Journal of Clinical Medicine. 2023; 12(17):5630. https://doi.org/10.3390/jcm12175630
Chicago/Turabian StyleDainese, Cristina, Federica Valeri, Benedetto Bruno, and Alessandra Borchiellini. 2023. "Anti-ADAMTS13 Autoantibodies: From Pathophysiology to Prognostic Impact—A Review for Clinicians" Journal of Clinical Medicine 12, no. 17: 5630. https://doi.org/10.3390/jcm12175630