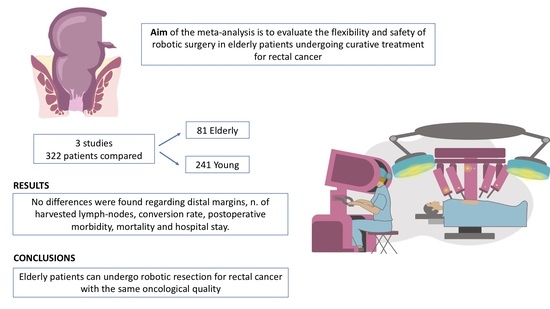
Robotic Rectal Resection for Rectal Cancer in Elderly Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection
2.2. Statistical Analysis
2.3. Heterogeneity
2.4. Quality Assessment
3. Results
3.1. Literature Selection
3.2. Study and Patient Characteristics
3.3. Risk of Bias
3.4. Oncologic Surgical Quality
3.5. Postoperative Outcomes
3.6. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv263, Erratum in Ann. Oncol. 2017, 28, iv22–v40. https://doi.org/10.1093/annonc/mdy161. [Google Scholar] [CrossRef]
- Kunitake, H.; Zingmond, D.S.; Ryoo, J.; Ko, C.Y. Caring for Octogenarian and Nonagenarian Patients with Colorectal Cancer: What Should Our Standards and Expectations Be? Dis. Colon Rectum 2010, 53, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Salinas, H.M.; Dursun, A.; Klos, C.L.; Shellito, P.; Sylla, P.; Berger, D.; Bordeianou, L. Determining the Need for Radical Surgery in Patients With T1 Rectal Cancer. Arch. Surg. 2011, 146, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.B.A.; Nahas, C.S.R.; Araujo, S.E.A.; Nahas, S.C.; Marques, C.F.S.; Kiss, D.R.; Cecconello, I. Early Rectal Cancer: Local Excision or Radical Surgery? J. Surg. Educ. 2008, 65, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nivatvongs, S. Surgical Management of Early Colorectal Cancer. World J. Surg. 2000, 24, 1052–1055. [Google Scholar] [CrossRef]
- Benson, A.B.; Abrams, T.A.; Ben-Josef, E.; Bloomston, P.M.; Botha, J.F.; Clary, B.M.; Covey, A.; Curley, S.A.; D’Angelica, M.I.; Davila, R. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). NCCN Evidence Blocks TM Rectal Cancer. 2023. Available online: www.nccn.org/patents (accessed on 25 March 2023).
- Nagtegaal, I.D.; Quirke, P. What Is the Role for the Circumferential Margin in the Modern Treatment of Rectal Cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique—Past, Present, and Future. Clin. Colon Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef]
- Heald, R.J. The ‘Holy Plane’ of Rectal Surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; van de Velde, C.J.; van der Worp, E.; Kapiteijn, E.; Quirke, P.; van Krieken, J.H.J. The Pathology Review Committee for the Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group Macroscopic Evaluation of Rectal Cancer Resection Specimen: Clinical Significance of the Pathologist in Quality Control. J. Clin. Oncol. 2002, 20, 1729–1734. [Google Scholar] [CrossRef]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef]
- Baek, S.-J.; Piozzi, G.N.; Kim, S.-H. Optimizing outcomes of colorectal cancer surgery with robotic platforms. Surg. Oncol. 2021, 37, 101559. [Google Scholar] [CrossRef]
- Safiejko, K.; Tarkowski, R.; Koselak, M.; Juchimiuk, M.; Tarasik, A.; Pruc, M.; Smereka, J.; Szarpak, L. Robotic-Assisted vs. Standard Laparoscopic Surgery for Rectal Cancer Resection: A Systematic Review and Meta-Analysis of 19,731 Patients. Cancers 2021, 14, 180. [Google Scholar] [CrossRef]
- Hurria, A.; Dale, W.; Mooney, M.; Rowland, J.H.; Ballman, K.V.; Cohen, H.J.; Muss, H.B.; Schilsky, R.L.; Ferrell, B.; Extermann, M.; et al. Designing Therapeutic Clinical Trials for Older and Frail Adults with Cancer: U13 Conference Recommendations. J. Clin. Oncol. 2014, 32, 2587–2594. [Google Scholar] [CrossRef]
- Fu, J.; Ruan, H.; Zheng, H.; Cai, C.; Zhou, S.; Wang, Q.; Chen, W.; Fu, W.; Du, J. Impact of old age on resectable colorectal cancer outcomes. PeerJ 2019, 7, e6350. [Google Scholar] [CrossRef]
- Podda, M.; Sylla, P.; Baiocchi, G.; Adamina, M.; Agnoletti, V.; Agresta, F.; Ansaloni, L.; Arezzo, A.; Avenia, N.; Biffl, W.; et al. Multidisciplinary management of elderly patients with rectal cancer: Recommendations from the SICG (Italian Society of Geriatric Surgery), SIFIPAC (Italian Society of Surgical Pathophysiology), SICE (Italian Society of Endoscopic Surgery and new technologies), and the WSES (World Society of Emergency Surgery) International Consensus Project. World J. Emerg. Surg. 2021, 16, 1–38. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Oldani, A.; Bellora, P.; Monni, M.; Amato, B.; Gentilli, S. Colorectal surgery in elderly patients: Our experience with DaVinci Xi® System. Aging Clin. Exp. Res. 2017, 29, 91–99. [Google Scholar] [CrossRef]
- Ramallo Solis, I.; Jimenez-Rodriguez, R.M.; Reyes-Diaz, M.L.; Diaz-Pavon, J.M.; Vazquez-Monchul, J.M.; Garcia-Cabrera, A.M.; Padillo, J.; de la Portilla, F. Influence of Robotics in Surgical Complication Rate in Elderly Population with Rectal Cancer. Aging Clin. Exp. Res. 2020, 32, 1585–1589. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Su, W.-C.; Huang, C.-W.; Ma, C.-J.; Chen, P.-J.; Tsai, H.-L.; Chang, T.-K.; Chen, Y.-C.; Li, C.-C.; Yeh, Y.-S. Feasibility of robot-assisted surgery in elderly patients with rectal cancer. J. Minimal Access Surg. 2021, 17, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Baili, P.; Capocaccia, R.; Caldora, M.; Carrani, E.; Minicozzi, P.; Pierannunzio, D.; Santaquilani, M.; Trama, A.; Allemani, C.; et al. The EUROCARE-5 study on cancer survival in Europe 1999–2007: Database, quality checks and statistical analysis methods. Eur. J. Cancer 2015, 51, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lawler, M.; Selby, P.; Aapro, M.S.; Duffy, S. Ageism in cancer care. BMJ 2014, 348, g1614. [Google Scholar] [CrossRef] [PubMed]
- Montroni, I.; Ugolini, G.; Saur, N.M.; Spinelli, A.; Rostoft, S.; Millan, M.; Wolthuis, A.; Daniels, I.R.; Hompes, R.; Penna, M.; et al. Personalized management of elderly patients with rectal cancer: Expert recommendations of the European Society of Surgical Oncology, European Society of Coloproctology, International Society of Geriatric Oncology, and American College of Surgeons Commission on Cancer. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1685–1702. [Google Scholar] [CrossRef]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs. Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): Short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 991–1004. [Google Scholar] [CrossRef]
- Goligher, J.C.; E Dukes, C.; Bussey, H.J.R. Local recurrences after sphincter-saving excisions for carcinoma of the rectum and rectosigmoid. Br. J. Surg. 2005, 39, 199–211. [Google Scholar] [CrossRef]
- Park, I.J.; Kim, J.C. Adequate Length of the Distal Resection Margin in Rectal Cancer: From the Oncological Point of View. J. Gastrointest. Surg. 2010, 14, 1331–1337. [Google Scholar] [CrossRef]
- Yan, H.; Wang, P.-Y.; Wu, Y.-C.; Liu, Y.-C. Is a Distal Resection Margin of ≤ 1 cm Safe in Patients with Intermediate- to Low-Lying Rectal Cancer? A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2022, 26, 1791–1803. [Google Scholar] [CrossRef]
- Gao, P.; Song, Y.; Yang, Y.; Zhao, S.; Sun, Y.; Sun, J.; Chen, X.; Wang, Z. What Is the Minimum Number of Examined Lymph Nodes After Neoadjuvant Therapy in Rectal Cancer? J. Gastrointest. Surg. 2018, 22, 1068–1076. [Google Scholar] [CrossRef]
- Degiuli, M.; Arolfo, S.; Evangelista, A.; Lorenzon, L.; Reddavid, R.; Staudacher, C.; De Nardi, P.; Rosati, R.; Elmore, U.; Coco, C.; et al. Number of lymph nodes assessed has no prognostic impact in node-negative rectal cancers after neoadjuvant therapy. Results of the “Italian Society of Surgical Oncology (S.I.C.O.) Colorectal Cancer Network” (SICO-CCN) multicentre collaborative study. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1233–1240. [Google Scholar] [CrossRef]
- Baxter, N.N.; Virnig, D.J.; Rothenberger, D.A.; Morris, A.M.; Jessurun, J.; Virnig, B.A. Lymph Node Evaluation in Colorectal Cancer Patients: A Population-Based Study. Gynecol. Oncol. 2005, 97, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mekenkamp, L.J.; van Krieken, J.H.J.M.; Marijnen, C.A.M.; van de Velde, C.J.H.; Nagtegaal, I.D. Lymph Node Retrieval in Rectal Cancer is Dependent on Many Factors—the Role of the Tumor, the Patient, the Surgeon, the Radiotherapist, and the Pathologist. Am. J. Surg. Pathol. 2009, 33, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Feroci, F.; Vannucchi, A.; Bianchi, P.P.; Cantafio, S.; Garzi, A.; Formisano, G.; Scatizzi, M. Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J. Gastroenterol. 2016, 22, 3602–3610. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, M.J.; Park, S.C.; Sohn, D.K.; Kim, D.Y.; Chang, H.J.; Nam, B.-H.; Oh, J.H. Robotic Versus Laparoscopic Surgery for Rectal Cancer after Preoperative Chemoradiotherapy: Case-Matched Study of Short-Term Outcomes. Cancer Res. Treat. 2016, 48, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, U.; Späth, C.; Müller, T.C.; Maak, M.; Janssen, K.-P.; Wilhelm, D.; Kleeff, J.; Bader, F.G. Colorectal cancer surgery remains effective with rising patient age. Int. J. Color. Dis. 2014, 29, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, E.; Fan, Z.; Birkmeyer, J.D. Outcomes in Octogenarians Undergoing High-Risk Cancer Operation: A National Study. J. Am. Coll. Surg. 2007, 205, 729–734. [Google Scholar] [CrossRef]
- Al-Refaie, W.B.; Parsons, H.M.; Habermann, E.B.; Kwaan, M.; Spencer, M.P.; Henderson, W.G.; Rothenberger, D.A. Operative Outcomes Beyond 30-day Mortality. Ann. Surg. 2011, 253, 947–952. [Google Scholar] [CrossRef]
- Duron, J.-J.; Duron, E.; Dugue, T.; Pujol, J.; Muscari, F.; Collet, D.; Pessaux, P.; Hay, J.-M. Risk Factors for Mortality in Major Digestive Surgery in the Elderly. Ann. Surg. 2011, 254, 375–382. [Google Scholar] [CrossRef]
- De’Angelis, N.; Abdalla, S.; Bianchi, G.; Memeo, R.; Charpy, C.; Petrucciani, N.; Sobhani, I.; Brunetti, F. Robotic Versus Laparoscopic Colorectal Cancer Surgery in Elderly Patients: A Propensity Score Match Analysis. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1334–1345. [Google Scholar] [CrossRef]

| Study | Country | Study Design | Patients | Mean Age (SD) | Sex (M/F) | ASA (I/II/III/IV) | Neoadjuvant Therapy Yes/No | cT Stage (T1/T2/T3/T4) | cN Stage (N0/N1/N2) | cM Stage (M0/M1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Oldani [19] 2017 | Italy | Retrospective study | 7 young | 57.12 (5.5) | 4M/3F | 1/6/0/0 | 4/3 | ND | ND | 6/1 |
| 8 elderly | 81.9 (6.28) | 4M/4F | 0/2/6/0 | 2/6 | ND | ND | 7/1 | |||
| Ramallo-Solis [20] 2019 | Spain | Retrospective analysis of a prospective database | 108 young | 60 (7.69) | 65M/43F | 12/69/27/0 | 71/37 | 4/25/78/1 | 41/41/26 | 102/6 |
| 43 elderly | 74.74 (3.566) | 29M/14F | 0/15/28/0 | 24/19 | 1/11/30/1 | 14/20/9 | 43/0 | |||
| Su [21] 2021 | Taiwan | Retrospective study | 126 young | 56.7 (7.9) | 77M/49F | 0/96/30/0 | 90/80 | 4/26/81/15 | 55/45/26 | ND |
| 30 elderly | 77.7 (5.63) | 17M/13F | 0/7/22/1 | 22/8 | ¼/23/2 | 13/16/1 | ND | |||
| Tot. | 241 young | 58,19 (7.11) | 144M/95F | 13/171/57/0 | 143/111 | |||||
| 81 elderly | 76,54 (5.29) | 50M/31F | 0/24/56/1 | 69/43 |
| Study | Representativeness of Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Absence of Outcome of Interest at Start of Study | Comparability of Cohorts on the Basis of Design or Analysis | Assessment of Outcome | Follow-Up Enough for Outcome to Occur | Adequacy of Follow-Up of Cohorts | Score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Oldani [19] | * | * | * | * | ** | * | * | - | 8 | Good |
| Ramallo-Solis [20] | * | * | * | * | ** | * | * | - | 8 | Good |
| Su [21] | * | * | * | * | ** | * | * | - | 8 | Good |
| Outcome | N of Studies | Means (SD) | Heterogeneity of Trials | p-Value for Differences across Studies | Mean Difference (C.I. 95%) | ||
|---|---|---|---|---|---|---|---|
| p-Value | I2 Statistic | ||||||
| Distal margin | 3 | Young 2.93 (0.46) | Elderly 2.37 (0.78) | 0.07 | 62% | 0.52 | −0.47 (−1.92, 0.97) |
| N. of harvested lymph nodes | 3 | Young 14.02 (1.83) | Elderly 12.70 (2.31) | 0.96 | 0% | 0.16 | −1.51 (−3.62, 0.59) |
| Outcome | No. of Studies | No. of Events or Mean (SD) | Heterogeneity of Trials | p-Value for Differences across Studies | OR, Mean or Risk Difference (C.I. 95%) | ||
|---|---|---|---|---|---|---|---|
| p-Value | I2 Statistic | ||||||
| Conversion rate | 3 | Young 14/241 | Elderly 7/81 | 0.73 | 0% | 0.63 | 0.02 (−0.05, 0.09) |
| Length of stay (LOS) | 3 | Young 13.19 (1.56) | Elderly 16.29 (4.08) | 0.28 | 22% | 0.56 | 1.23 (−2.91, 5.38) |
| Overall complications | 3 | Young 56/241 | Elderly 17/81 | 0.68 | 0% | 0.71 | 0.89 (0.48, 1.65) |
| Postoperative mortality | 3 | Young 0/241 | Elderly 0/81 | 1.00 | 0% | 1.00 | 0.00 (−0.03, 0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddavid, R.; Sofia, S.; Puca, L.; Moro, J.; Ceraolo, S.; Jimenez-Rodriguez, R.; Degiuli, M. Robotic Rectal Resection for Rectal Cancer in Elderly Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5331. https://doi.org/10.3390/jcm12165331
Reddavid R, Sofia S, Puca L, Moro J, Ceraolo S, Jimenez-Rodriguez R, Degiuli M. Robotic Rectal Resection for Rectal Cancer in Elderly Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(16):5331. https://doi.org/10.3390/jcm12165331
Chicago/Turabian StyleReddavid, Rossella, Silvia Sofia, Lucia Puca, Jacopo Moro, Simona Ceraolo, Rosa Jimenez-Rodriguez, and Maurizio Degiuli. 2023. "Robotic Rectal Resection for Rectal Cancer in Elderly Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 16: 5331. https://doi.org/10.3390/jcm12165331