Antiphospholipid Antibodies and Heart Failure with Preserved Ejection Fraction. The Multicenter ATHERO-APS Study
Abstract
:1. Introduction
2. Materials and Methods
3. NT-ProBNP Measurement
4. Transthoracic Echocardiography
5. Ethical Statement
6. Statistical Analysis
7. Results
Characteristics of Patients with HFpEF
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pignatelli, P.; Ettorre, E.; Menichelli, D.; Pani, A.; Violi, F.; Pastori, D. Seronegative antiphospholipid syndrome: Refining the value of “non-criteria” antibodies for diagnosis and clinical management. Haematologica 2020, 105, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Serrano, R.; Pons-Estel, G.J.; Espinosa, G.; Quintana, R.M.; Reverter, J.C.; Tassies, D.; Monteagudo, J.; Cervera, R. Long-term follow-up of antiphospholipid syndrome: Real-life experience from a single center. Lupus 2020, 29, 1050–1059. [Google Scholar] [CrossRef]
- Merashli, M.; Ster, I.C.; D’Andrea, G.; Iannaccone, L.; Marottoli, V.; Margaglione, M.; Brancaccio, V.; Ames, P.R.J. Survival in primary antiphospholipid syndrome. Thromb. Haemost. 2016, 115, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
- Radin, M.; Schreiber, K.; Cecchi, I.; Roccatello, D.; Cuadrado, M.J.; Sciascia, S. The risk of ischaemic stroke in primary antiphospholipid syndrome patients: A prospective study. Eur. J. Neurol. 2017, 25, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Taraborelli, M.; Reggia, R.; Dall’Ara, F.; Fredi, M.; Andreoli, L.; Gerosa, M.; Hoxha, A.; Massaro, L.; Tonello, M.; Costedoat-Chalumeau, N.; et al. Longterm Outcome of Patients with Primary Antiphospholipid Syndrome: A Retrospective Multicenter Study. J. Rheumatol. 2017, 44, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Merashli, M.; Bucci, T.; Pastori, D.; Pignatelli, P.; Marottoli, V.; Arcaro, A.; Gentile, F.; Ames, P.R. Antiphospholipid antibodies and lower extremity peripheral artery disease: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020, 50, 1291–1298. [Google Scholar] [CrossRef]
- Tufano, A.; Lembo, M.; Di Minno, M.N.; Nardo, A.; Esposito, R.; Santoro, C.; Buonauro, A.; Cerbone, A.M.; Di Minno, G.; Galderisi, M. Left ventricular diastolic abnormalities other than valvular heart disease in antiphospholipid syndrome: An echocardiographic study. Int. J. Cardiol. 2018, 271, 366–370. [Google Scholar] [CrossRef]
- Djokovic, A.; Stojanovich, L.; Kontic, M.; Stanisavljevic, N.; Radovanovic, S.; Marisavljevic, D. Association between cardiac manifestations and antiphospholipid antibody type and level in a cohort of Serbian patients with primary and secondary antiphospholipid syndrome. Isr. Med. Assoc. J. IMAJ 2014, 16, 162–167. [Google Scholar]
- Vaccaro, F.; Caccavo, D.; Roumpedaki, E.; De Vincentis, G.; Di Gioia, C.; Gallo, P.; Palange, P. Dilated Cardiomyopathy Due to Thrombotic Microangiopathy as the only Manifestation of Antiphospholipid Syndrome: A Case Report. Int. J. Immunopathol. Pharmacol. 2008, 21, 237–241. [Google Scholar] [CrossRef]
- Schrage, B.; Geelhoed, B.; Niiranen, T.J.; Gianfagna, F.; Vishram-Nielsen, J.K.K.; Costanzo, S.; Söderberg, S.; Ojeda, F.M.; Vartiainen, E.; Donati, M.B.; et al. Comparison of Cardiovascular Risk Factors in European Population Cohorts for Predicting Atrial Fibrillation and Heart Failure, Their Subsequent Onset, and Death. J. Am. Heart Assoc. 2020, 9, e015218. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Rehman, S.U.; Mohammed, A.A.; Bhardwaj, A.; Barajas, L.; Barajas, J.; Kim, H.-N.; Baggish, A.L.; Weiner, R.B.; Chen-Tournoux, A.; et al. Use of Amino-Terminal Pro–B-Type Natriuretic Peptide to Guide Outpatient Therapy of Patients with Chronic Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2011, 58, 1881–1889; [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Bhatia, R.S.; Tu, J.; Lee, D.; Austin, P.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. N. Engl. J. Med. 2006, 355, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Anavekar, N.S.; Senni, M.; Gori, M.; Jhund, P.C.; McGrath, M.M.; Packer, M.; Shi, V.; et al. Angiotensin-Neprilysin Inhibition and Renal Outcomes in Heart Failure with Preserved Ejection Fraction. Circulation 2020, 142, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, E.D.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2019, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens 2018, 36, 1953–2041. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; De Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [Green Version]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Pan, J.; Lin, H.; Wang, C.; Zhang, J.; Gu, J. Optimal management of blood glucose, blood pressure and atrial fibrillation to reduce the risk of heart failure with preserved ejection fraction. Intern. Med. J. 2020. [CrossRef]
- Pastori, D.; Bucci, T.; Triggiani, M.; Ames, P.R.; Parrotto, S.; Violi, F.; Pignatelli, P.; Farcomeni, A. Immunoglobulin G (IgG) anticardiolipin antibodies and recurrent cardiovascular events. A systematic review and Bayesian meta-regression analysis. Autoimmun. Rev. 2019, 18, 519–525. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Khamashta, M.A.; Haworth, R.S.; Brooks, G.; Amengual, O.; Ichikawa, K.; Koike, T.; Hughes, G.R.V. Arterial disease and thrombosis in the antiphospholipid syndrome: A pathogenic role for endothelin 1. Arthritis Rheum. 1998, 41, 800–807. [Google Scholar] [CrossRef]
- Merashli, M.; Bucci, T.; Pastori, D.; Pignatelli, P.; Arcaro, A.; Gentile, F.; Marottoli, V.; Ames, P.R. Isoprostanes in systemic lupus erythematosus and antiphospholipid syndrome: A systematic review and meta-analysis. Autoimmun. Rev. 2021, 20, 102821. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.; Batuca, J.R.; Ciampa, A.; Iannaccone, L.; Alves, J.D. Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome. J. Rheumatol. 2010, 37, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.D.; Ames, P.R. Atherosclerosis, oxidative stress and auto-antibodies in systemic lupus erythematosus and primary antiphospholipid syndrome. Immunobiology 2003, 207, 23–28. [Google Scholar] [CrossRef]
- Stanisavljevic, N.; Stojanovich, L.; Marisavljevic, D.; Djokovic, A.; Dopsaj, V.; Kotur-Stevuljevic, J.; Martinovic, J.; Memon, L.; Radovanovic, S.; Todic, B.; et al. Lipid peroxidation as risk factor for endothelial dysfunction in antiphospholipid syndrome patients. Clin. Rheumatol. 2016, 35, 2485–2493. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Carnevale, R.; Violi, F. Nox-2 up-regulation and platelet activation: Novel insights. Prostaglandins Other Lipid Mediat. 2015, 120, 50–55. [Google Scholar] [CrossRef]
- Weaver, J.C.; Krilis, S.A.; Giannakopoulos, B. Oxidative post-translational modification of betaeta 2-glycoprotein I in the pathophysiology of the anti-phospholipid syndrome. Free Radic. Biol. Med. 2018, 125, 98–103. [Google Scholar] [CrossRef]
- Sciacqua, A.; Borrello, F.; Vatrano, M.; Grembiale, R.D.; Perticone, F. Effect of interaction between left ventricular dysfunction and endothelial function in hypertension. Curr. Hypertens. Rep. 2006, 8, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Duarte-Garcia, A.; McBane, R.D.; Matteson, E.L.; Lerman, L.O.; Lerman, A. Antiphospholipid Syndrome: Role of Vascular Endothelial Cells and Implications for Risk Stratification and Targeted Therapeutics. J. Am. Coll. Cardiol. 2017, 69, 2317–2330. [Google Scholar] [CrossRef]
- Sobirin, M.A.; Herry, Y.; Sofia, S.N.; Uddin, I.; Rifqi, S.; Tsutsui, H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discov. Ther. 2019, 13, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, C.; Aguirre, M.Á.; Ruiz-Limón, P.; Ábalos-Aguilera, M.C.; Jiménez-Gómez, Y.; Arias-de la Rosa, I.; Rodriguez-Ariza, A.; Fernández-del Río, L.; González-Reyes, J.A.; Segui, P.; et al. Ubiquinol Effects on Antiphospholipid Syndrome Prothrombotic Profile: A Randomized, Placebo-Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Ames, P.R.J.; Tommasino, C.; Alves, J.D.; Morrow, J.D.; Iannaccone, L.; Fossati, G.; Caruso, S.; Caccavo, F.; Brancaccio, V.; Ames, P.R.J.; et al. Antioxidant susceptibility of pathogenic pathways in subjects with antiphospholipid antibodies: A pilot study. Lupus 2000, 9, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.J.; Merashli, M.; Tommaso, B.; Iannaccone, L.; Marottoli, V.; Ciampa, A. Intensity of immune/clotting assays relate to multiple antiphospholipid antibody positivity in thrombotic primary antiphospholipid syndrome. Int. J. Hematol. 2021, 113, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Gresele, P.; Barcellona, D.; Erba, N.; Testa, S.; Marongiu, F.; Bison, E.; Denas, G.; et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010, 8, 237–242. [Google Scholar] [CrossRef]
- Zuily, S.; Clerc-Urmès, I.; Bauman, C.; Andrade, D.; Sciascia, S.; Pengo, V.; Tektonidou, M.G.; Ugarte, A.; Gerosa, M.; Belmont, H.M.; et al. Cluster analysis for the identification of clinical phenotypes among antiphospholipid antibody-positive patients from the APS ACTION Registry. Lupus 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Radin, M.; Cecchi, I.; Levy, R.A.; Erkan, D. 16th International congress on antiphospholipid antibodies task force report on clinical manifestations of antiphospholipid syndrome. Lupus 2021, 30, 1314–1326. [Google Scholar] [CrossRef]
| PAPS (n = 91) | APS-SLE (n = 18) | Carriers (n = 16) | p among Groups | |
|---|---|---|---|---|
| Age (years) | 51.4 ± 14.0 | 52.2 ± 14.8 | 47.2 ± 14.1 | 0.501 # |
| Women (%) | 60 (65.9) | 16 (88.9) | 13 (81.3) | 0.092 § |
| Hypertension (%) | 42 (46.2) | 14 (77.8) | 4 (25.0) | 0.007 § |
| Diabetes (%) | 3 (3.3) | 3 (16.7) | 0 (0.0) | 0.033 § |
| Smoking (%) | 21 (23.1) | 2 (11.1) | 1 (6.3) | 0.185 § |
| Previous arterial events (%) | 31 (34.1) | 8 (44.4) | 0 (0.0) | 0.011 § |
| Previous VTE (%) | 65 (71.4) | 9 (50.0) | 0 (0.0) | <0.001 § |
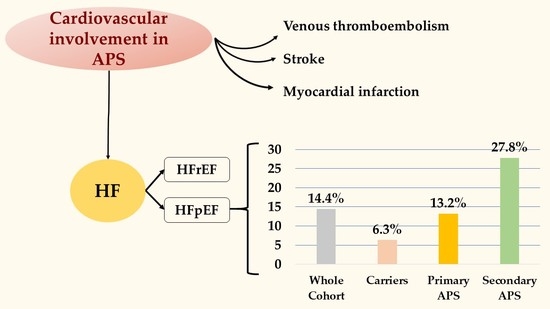
| HFpEF (%) | 12 (13.2) | 5 (27.8) | 1 (6.3) | 0.167 § |
| NT-ProBNP (pg/mL) | 455.9 ± 118.6 * | 537.1 ± 116.0 ** | 288.2 ± 41.0 | <0.001 # |
| Treatments | ||||
| Hydroxychloroquine (%) | 13 (14.3) | 8 (44.4) | 5 (31.3) | 0.009 § |
| Proton pump inhibitors (%) | 23 (25.3) | 11 (61.1) | 4 (25.0) | 0.009 § |
| Corticosteroids (%) | 12 (13.2) | 14 (77.8) | 5 (31.3) | <0.001 § |
| Antiplatelet drugs (%) | 18 (19.8) | 3 (16.7) | 8 (50.0) | 0.024 § |
| Oral anticoagulants (%) | 60 (65.9) | 10 (55.6) | 0 (0.0) | <0.001 § |
| Statins (%) | 20 (22.0) | 0 (0.0) | 2 (12.5) | 0.069 § |
| ACEi/ARBs (%) | 33 (36.3) | 10 (55.6) | 3 (18.8) | 0.083 § |
| Beta blockers (%) | 21 (23.1) | 6 (33.3) | 2 (12.5) | 0.356 § |
| Calcium channel blockers (%) | 8 (8.9) | 6 (33.3) | 0 (0.0) | 0.004 § |
| Diuretics (%) | 17 (18.7) | 2 (11.1) | 2 (12.5) | 0.651 § |
| Autoantibodies | ||||
| aCL IgG > 40 GPL (%) | 41 (46.1) | 6 (33.3) | 4 (25.0) | 0.217 § |
| aCL IgM > 40 MPL (%) | 15 (16.9) | 3 (16.7) | 4 (25.0) | 0.728 § |
| aβ2GPI IgG > 40 GPL (%) | 36 (40.9) | 8 (44.4) | 6 (37.5) | 0.919 § |
| aβ2GPI IgM > 40 MPL (%) | 11 (12.5) | 2 (11.1) | 3 (18.8) | 0.764 § |
| LAC DRVVT > 1.25 (%) | 66 (74.2) | 12 (66.7) | 2 (12.5) | <0.001 § |
| Triple positivity (%) | 41 (46.1) | 6 (33.3) | 1 (6.3) | 0.009 § |
| Echocardiography measurements | ||||
| Ejection fraction (%) | 60.1 ± 7.2 | 56.9 ± 6.3 | 61.7 ± 6.1 | 0.122 # |
| LVED volume/BSA (mL/m2) | 53.1 ± 13.2 | 54.7 ± 9.8 | 52.7 ± 9.7 | 0.881 # |
| LVM/BSA (g/m2) | 81.2 ± 22.9 | 80.7 ± 20.3 | 77.7 ± 28.5 | 0.861 # |
| Normal LV geometry (%) | 54.4 | 55.6 | 68.8 | 0.929 § |
| Concentric remodeling (%) | 25.6 | 22.2 | 18.8 | |
| Concentric hypertrophy (%) | 8.9 | 5.6 | 6.3 | |
| Eccentric hypertrophy (%) | 11.1 | 16.7 | 6.3 | |
| LA diameter (mm) | 35.9 ± 5.9 | 38.8 ± 6.6 | 32.7 ± 4.4 | 0.012 # |
| LA area (cm2) | 18.9 ± 4.3 | 20.3 ± 6.2 | 17.2 ± 3.5 | 0.166 # |
| LA volume/BSA (mL/m2) | 28.7 ± 8.6 | 32.7 ± 12.8 | 22.9 ± 7.4 | 0.010 # |
| E/A ratio | 1.22 ± 0.49 | 1.04 ± 0.32 | 1.25 ± 0.48 | 0.359 # |
| e’ septal | 0.09 ± 0.03 | 0.09 ± 0.02 | 0.10 ± 0.02 | 0.543 # |
| e’ lateral | 0.11 ± 0.04 | 0.11 ± 0.04 | 0.11 ± 0.03 | 0.947 # |
| Mean E/e’ ratio | 7.80 ± 2.49 | 9.09 ± 3.27 | 7.35 ± 1.27 | 0.103 # |
| HFpEF No (n = 107) | HFpEF Yes (n = 18) | p among Groups | |
|---|---|---|---|
| PAPS (%) | 79 (73.8) | 12 (66.7) | 0.167 § |
| APS-SLE (%) | 13 (12.1) | 5 (27.8) | |
| Carriers (%) | 15 (14.0) | 1 (5.6) | |
| Age (years) | 49.8 ± 14.3 | 58.3 ± 10.1 | 0.017 # |
| Women (%) | 79 (73.8) | 10 (55.6) | 0.113 § |
| Hypertension (%) | 43 (40.2) | 17 (94.4) | <0.001 |
| Diabetes (%) | 4 (3.7) | 2 (11.1) | 0.176 § |
| Smoking (%) | 21 (19.6) | 3 (16.7) | 0.768 § |
| Previous arterial events (%) | 29 (27.1) | 10 (55.6) | 0.016 § |
| Previous VTE (%) | 67 (62.6) | 7 (38.9) | 0.058 § |
| Treatments | |||
| Hydroxychloroquine (%) | 19 (17.8) | 7 (38.9) | 0.041 § |
| Proton pump inhibitors (%) | 31 (29.0) | 7 (38.9) | 0.397 § |
| Corticosteroids (%) | 24 (22.4) | 7 (38.9) | 0.135 § |
| Antiplatelet drugs (%) | 23 (21.5) | 6 (33.3) | 0.271 § |
| Oral anticoagulants (%) | 61 (57.0) | 9 (50.0) | 0.579 § |
| Statins (%) | 17 (15.9) | 5 (27.8) | 0.220 § |
| ACEi/ARBs(%) | 34 (31.5) | 12 (66.7) | 0.005 § |
| Beta blockers (%) | 17 (15.9) | 12 (66.7) | <0.001 § |
| Calcium channel antagonists (%) | 9 (8.5) | 5 (27.8) | 0.017 § |
| Diuretics (%) | 14 (13.1) | 7 (38.9) | 0.007 § |
| Autoantibodies | |||
| aCL IgG > 40 GPL (%) * | 40 (37.7) | 11 (64.7) | 0.036 § |
| aCL IgM > 40 MPL (%) * | 18 (17.0) | 4 (23.5) | 0.513 § |
| aβ2GPI IgG > 40 GPL (%) * | 39 (37.1) | 11 (64.7) | 0.032 § |
| aβ2GPI IgM > 40 MPL (%) * | 11 (10.5) | 5 (29.4) | 0.032 § |
| LAC DRVVT > 1.25 (%) * | 65 (61.3) | 15 (88.2) | 0.031 § |
| Triple positivity (%) * | 38 (35.8) | 10 (58.8) | 0.071 § |
| Echocardiography measurements | |||
| Ejection fraction (%) | 60.7 ± 6.4 | 55.1 ± 8.6 | 0.001 # |
| LVED volume/BSA (mL/m2) | 52.1 ± 12.1 | 60.1 ± 11.7 | 0.011 # |
| LVM/BSA (g/m2) | 75.7 ± 18.8 | 109.8 ± 26.1 | <0.001 # |
| Normal LV geometry (%) | 59.8 | 35.3 | <0.001 § |
| Concentric remodeling (%) | 26.2 | 11.8 | |
| Concentric hypertrophy (%) | 7.5 | 11.8 | |
| Eccentric hypertrophy (%) | 6.5 | 41.2 | |
| LA diameter (mm) | 34.6 ± 5.3 | 43.4 ± 4.1 | <0.001 # |
| LA area (cm2) | 17.7 ± 3.6 | 25.3 ± 3.8 | <0.001 # |
| LA volume/BSA (mL/m2) | 26.3 ± 7.6 | 40.9 ± 9.1 | <0.001 # |
| E/A ratio | 1.22 ± 0.48 | 1.06 ± 0.40 | 0.184 # |
| e’ septal | 0.09 ± 0.03 | 0.07 ± 0.01 | <0.001 # |
| e’ lateral | 0.12 ± 0.04 | 0.08 ± 0.02 | <0.001 # |
| Mean E/e’ ratio | 7.22 ± 1.86 | 11.91 ± 2.07 | <0.001 # |
| Odds Ratio | 95% CI | p Value | ||
|---|---|---|---|---|
| PAPS * | Univariable | 2.28 | 0.27–18.86 | 0.445 |
| APS-SLE * | 5.77 | 0.60–55.95 | 0.131 | |
| PAPS * | Sex and age-adjusted | 1.49 | 0.17–13.44 | 0.721 |
| APS-SLE * | 5.93 | 0.56–63.07 | 0.140 | |
| Age | Univariable | 1.05 | 1.01–1.09 | 0.020 |
| Female sex | Univariable | 0.44 | 0.16–1.24 | 0.119 |
| Age | Sex and age-adjusted | 1.05 | 1.01–1.10 | 0.013 |
| Female sex | 0.37 | 0.12–1.08 | 0.067 | |
| Hypertension | Univariable | 25.3 | 3.3–197.2 | 0.002 |
| Sex and age-adjusted | 20.0 | 2.4–166.7 | 0.006 | |
| Diabetes | Univariable | 3.22 | 0.54–19.03 | 0.197 |
| Sex and age-adjusted | 1.81 | 0.29–11.16 | 0.522 | |
| Smoking | Univariable | 0.82 | 0.22–3.09 | 0.768 |
| Sex and age-adjusted | 0.92 | 0.23–3.64 | 0.903 | |
| Previous arterial events | Univariable | 3.36 | 1.21–9.35 | 0.020 |
| Sex and age-adjusted | 2.67 | 0.91–7.83 | 0.074 | |
| Previous VTE | Univariable | 0.38 | 0.14–1.06 | 0.064 |
| Sex and age-adjusted | 0.37 | 0.13–1.08 | 0.068 | |
| aCL IgG > 40 GPL | Univariable | 3.03 | 1.04–8.81 | 0.042 |
| Sex and age-adjusted | 3.43 | 1.09–10.77 | 0.035 | |
| aCL IgM > 40 MPL | Univariable | 1.50 | 0.44–5.15 | 0.515 |
| Sex and age-adjusted | 1.21 | 0.33–4.42 | 0.772 | |
| aβ2GPI IgG > 40 GPL | Univariable | 3.10 | 1.06–9.05 | 0.038 |
| Sex and age-adjusted | 5.28 | 1.53–18.27 | 0.009 | |
| aβ2GPI IgM > 40 MPL | Univariable | 3.56 | 1.06–12.01 | 0.041 |
| Sex and age-adjusted | 2.64 | 0.72–9.67 | 0.144 | |
| LAC DRVVT > 1.25 | Univariable | 4.73 | 1.03–21.77 | 0.046 |
| Sex and age-adjusted | 5.20 | 1.10–24.68 | 0.038 | |
| Triple positivity | Univariable | 2.56 | 0.90–7.26 | 0.078 |
| Sex and age-adjusted | 3.56 | 1.11–11.47 | 0.033 |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Age | 1.07 | 1.00–1.14 | 0.044 |
| Female sex | 0.50 | 0.13–1.85 | 0.298 |
| Arterial hypertension | 19.49 | 2.21–171.94 | 0.008 |
| aβ2GPI IgG > 40 GPL | 8.62 | 1.23–60.44 | 0.030 |
| aCL IgG > 40 GPL | 0.65 | 0.11–3.96 | 0.640 |
| LAC DRVVT > 1.25 | 2.57 | 0.43–15.26 | 0.298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastori, D.; Ames, P.R.J.; Triggiani, M.; Ciampa, A.; Cammisotto, V.; Carnevale, R.; Pignatelli, P.; Bucci, T.; on behalf of the ATHERO-APS Study Group. Antiphospholipid Antibodies and Heart Failure with Preserved Ejection Fraction. The Multicenter ATHERO-APS Study. J. Clin. Med. 2021, 10, 3180. https://doi.org/10.3390/jcm10143180
Pastori D, Ames PRJ, Triggiani M, Ciampa A, Cammisotto V, Carnevale R, Pignatelli P, Bucci T, on behalf of the ATHERO-APS Study Group. Antiphospholipid Antibodies and Heart Failure with Preserved Ejection Fraction. The Multicenter ATHERO-APS Study. Journal of Clinical Medicine. 2021; 10(14):3180. https://doi.org/10.3390/jcm10143180
Chicago/Turabian StylePastori, Daniele, Paul R. J. Ames, Massimo Triggiani, Antonio Ciampa, Vittoria Cammisotto, Roberto Carnevale, Pasquale Pignatelli, Tommaso Bucci, and on behalf of the ATHERO-APS Study Group. 2021. "Antiphospholipid Antibodies and Heart Failure with Preserved Ejection Fraction. The Multicenter ATHERO-APS Study" Journal of Clinical Medicine 10, no. 14: 3180. https://doi.org/10.3390/jcm10143180