Mussel-Inspired Surface Functionalization of AEM for Simultaneously Improved Monovalent Anion Selectivity and Antibacterial Property
Abstract
:1. Introduction
2. Experimental
2.1. Materials
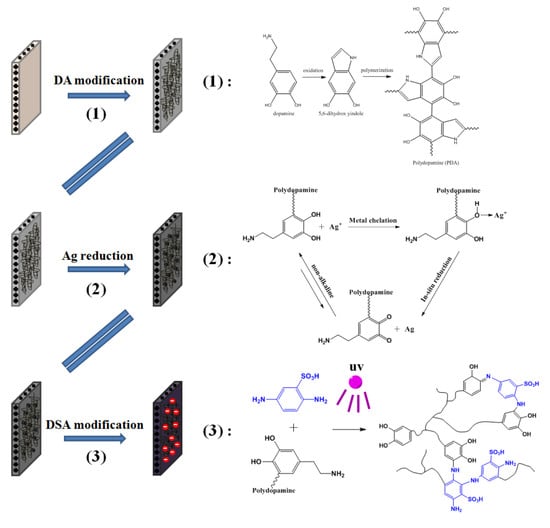
2.2. Membrane Modification
2.2.1. Synthesis of Silver Nanoparticles Chelated Dopamine Coating onto Membrane Surface
2.2.2. Grafting of Sulfonic Functional Groups on Prepared Membranes
2.3. FTIR Characterization and X-ray Photoelectron Spectroscopy (XPS)
2.4. Electrochemical Characterization of Modified AEMs
2.4.1. Membrane Surface Resistance and Ion Exchange Capability
2.4.2. ζ-Potential
2.5. Monovalent Anions Selectivity Measurement
2.6. Antibacterial Test of Membranes
2.6.1. Antibacterial Activity Test
2.6.2. Bacterial Suspension Test
3. Result and Discussion
3.1. Surface Characterization of the Membrane Surfaces
3.2. Membrane Surface Resistance and Ion Exchange Capability
3.3. ζ-Potential of Membrane Surface
3.4. Monovalent Anion Selectivity
3.5. Antibacterial Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Shalaby, S.M. Reverse osmosis desalination powered by photovoltaic and solar Rankine cycle power systems: A review. Renew. Sustain. Energy Rev. 2017, 73, 789–797. [Google Scholar] [CrossRef]
- Lee, H.; Jin, Y.; Hong, S. Recent transitions in ultrapure water (UPW) technology: Rising role of reverse osmosis (RO). Desalination 2016, 399, 185–197. [Google Scholar] [CrossRef]
- Deghles, A.; Kurt, U. Treatment of tannery wastewater by a hybrid electrocoagulation/electrodialysis process. Chem. Eng. Process. Process Intensif. 2016, 104, 43–50. [Google Scholar] [CrossRef]
- Strathmann, H.; Kedem, O.; Wilf, M. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Menkouchi Sahli, M.A.; Annouar, S.; Tahaikt, M.; Mountadar, M.; Soufiane, A.; Elmidaoui, A. Fluoride removal for underground brackish water by adsorption on the natural chitosan and by electrodialysis. Desalination 2007, 212, 37–45. [Google Scholar] [CrossRef]
- Bruggen, B.V.D. 6–Advances in electrodialysis for water treatment. Adv. Membr. Technol. Water Treat. 2015, 30, 185–203. [Google Scholar]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, M.; Jia, Y.X.; Wang, B.B. Surface Modification of Anion Exchange Membrane by Covalent Grafting for Imparting Permselectivity between Specific Anions. Electrochim. Acta 2015, 174, 1113–1121. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis—Experimental verification of theoretical predictions. J. Membr. Sci. 2015, 490, 301–310. [Google Scholar] [CrossRef]
- Liang, G.; Wu, L.; Wu, B.; Wang, G.; Xu, T. Preparation of monovalent cation selective membranes through annealing treatment. J. Membr. Sci. 2014, 459, 217–222. [Google Scholar]
- He, Y.; Xu, L.; Feng, X.; Zhao, Y.; Chen, L. Dopamine-Induced Nonionic Polymer Coatings for Significantly Enhancing Separation and Antifouling Properties of Polymer Membranes: Codeposition versus Sequential Deposition. J. Membr. Sci. 2017, 539, 421–431. [Google Scholar] [CrossRef]
- Sata, T. Studies on Anion Exchange Membranes Having Permselectivity for Specific Anions in Electrodialysis-effect of Hydrophilicity of Anion Exchange Membranes on Permselectivity of Anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Alemayehu, E.; Yaroshchuk, A.; Bruening, M.L. Highly selective separations of multivalent and monovalent cations in electrodialysis through Nafion membranes coated with polyelectrolyte multilayers. Polymer 2016, 103, 478–485. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Liu, H.; Bruggen, B.V.D.; Díaz, A.S.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Membr. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Pan, J.; Ding, J.; Tan, R.; Chen, G.; Zhao, Y.; Gao, C.; Bruggen, B.V.D.; Shen, J. Preparation of a monovalent selective anion exchange membrane through constructing a covalently crosslinked interface by electro-deposition of polyethyleneimine. J. Membr. Sci. 2017, 539, 263–272. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Membr. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, S.; Zhang, G.; Li, W.; Gao, C.; Shen, C.; Meng, Q. Antimicrobial polysulfone blended ultrafiltration membranes prepared with Ag/Cu2O hybrid nanowires. J. Membr. Sci. 2016, 509, 83–93. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, C.; Wang, Z.; Xu, Y.; Zhao, W.; Sun, S.; Zhao, C. Co-deposition towards mussel-inspired antifouling and antibacterial membranes by using zwitterionic polymers and silver nanoparticles. J. Mater. Chem. B 2017, 5, 7186–7193. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Liu, J.; Shi, Y.S.; Wang, Y.; Liu, H.F.; Hu, Q.L.; Su, L.; Zhu, J. Antifouling polyimide membrane with surface-bound silver particles. J. Membr. Sci. 2016, 516, 83–93. [Google Scholar] [CrossRef]
- Wu, J.; Yu, C.; Li, Q. Regenerable antimicrobial activity in polyamide thin film nanocomposite membranes. J. Membr. Sci. 2015, 476, 119–127. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Feng, X.; Wang, W.; Xu, F.; Zhang, L. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater Chem Phys. Mater. Chem. Phys. 2010, 121, 534–540. [Google Scholar]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, J.S.; Ryu, J.; Lee, S.H.; Lee, D.Y.; Kim, D.P.; Park, C.B.; Lee, H. Bio-inspired strategy for on-surface synthesis of silver nanoparticles for metal/organic hybrid nanomaterials and LDI-MS substrates. Nanotechnology 2011, 22, 494020. [Google Scholar] [CrossRef]
- Venault, A.; Yang, H.S.; Chiang, Y.C.; Lee, B.S.; Ruaan, R.C.; Chang, Y. Bacterial resistance control on mineral surfaces of hydroxyapatite and human teeth via surface charge-driven antifouling coatings. ACS Appl. Mater. Interfaces 2014, 6, 3201. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Zhang, K. Modification of PES membrane with Ag–SiO2: Reduction of biofouling and improvement of filtration performance. Desalination 2014, 336, 8–17. [Google Scholar] [CrossRef]
- Peng, L.; Guo, R.; Lan, J.; Jiang, S.; Lin, S. Microwave-assisted deposition of silver nanoparticles on bamboo pulp fabric through dopamine functionalization. Appl. Surf. Sci. 2016, 386, 151–159. [Google Scholar] [CrossRef]
- Hong, X.; Xue, S.; Hui, M.; Lv, Y.; Zhang, L.; Mao, Z. The preparation and antibacterial effects of dopa-cotton/AgNPs. Appl. Surf. Sci. 2011, 257, 6799–6803. [Google Scholar]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2010, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, K.; Ruan, H.; Xue, L.; Bruggen, B.V.D.; Gao, C.; Shen, J. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J. Membr. Sci. 2017, 536, 167–175. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, H.; Zhao, Y.; Pan, J.; Sotto, A.; Gao, C.; Bruggen, B.V.D.; Shen, J. A facile avenue to modify polyelectrolyte multilayers on anion exchange membranes to enhance monovalent selectivity and durability simultaneously. J. Membr. Sci. 2017, 543, 310–318. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; De, G.B.; Verstraete, W. Biogenic silver nanoparticles (bio-Ag 0) decrease biofouling of bio-Ag 0/PES nanocomposite membranes. Water Res. 2012, 46, 2077–2087. [Google Scholar] [CrossRef] [PubMed]












| Membrane Type | Thickness (μm) | Area Resistance (Ω·cm2) | pH Stability | Functional Group |
|---|---|---|---|---|
| Homogeneous (AEM-Type I) | 125 | 1.3 | 2–10 | Quaternary amino group |
| Homogeneous (CEM-Type II) | 135 | 2.7 | 4–12 | Sulfonic group |
| Type | Area Resistance (Ω·cm2) | IEC (mmol·g−1) | |
|---|---|---|---|
| in 0.5 M NaCl Solution | in 0.5 M Na2SO4 Solution | ||
| Pristine AEM | 1.03 ± 0.02 | 3.09 ± 0.03 | 1.71 ± 0.02 |
| DA/AEM | 1.27 ± 0.02 | 3.79 ± 0.06 | 1.67 ± 0.02 |
| DA/DSA/AEM | 1.67 ± 0.03 | 5.15 ± 0.05 | 1.55 ± 0.01 |
| DA/Ag/AEM | 1.28 ± 0.01 | 3.92 ± 0.04 | 1.66 ± 0.01 |
| DA/Ag/DSA/AEM | 1.49 ± 0.02 | 4.85 ± 0.05 | 1.54 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Bruggen, B.V.d.; Xiao, P.; Shen, J.; Ruan, H.; Liao, J.; Gao, C.; Bruggen, B.V.d.; Shen, J. Mussel-Inspired Surface Functionalization of AEM for Simultaneously Improved Monovalent Anion Selectivity and Antibacterial Property. Membranes 2019, 9, 36. https://doi.org/10.3390/membranes9030036
Zheng Z, Bruggen BVd, Xiao P, Shen J, Ruan H, Liao J, Gao C, Bruggen BVd, Shen J. Mussel-Inspired Surface Functionalization of AEM for Simultaneously Improved Monovalent Anion Selectivity and Antibacterial Property. Membranes. 2019; 9(3):36. https://doi.org/10.3390/membranes9030036
Chicago/Turabian StyleZheng, Zhihao, Bart Van der Bruggen, Pang Xiao, Jiangnan Shen, Huimin Ruan, Junbin Liao, Congjie Gao, Bart Van der Bruggen, and Jiangnan Shen. 2019. "Mussel-Inspired Surface Functionalization of AEM for Simultaneously Improved Monovalent Anion Selectivity and Antibacterial Property" Membranes 9, no. 3: 36. https://doi.org/10.3390/membranes9030036