Controlling Fractional Free Volume, Transport, and Co-Transport of Alcohols and Carboxylate Salts in PEGDA Membranes
Abstract
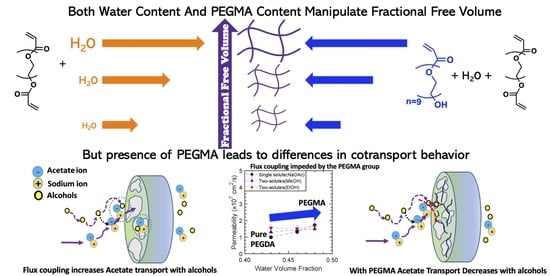
:1. Introduction
2. Materials and Methods
2.1. Materials, Film Formation, and Physiochemical Parameters Measurement
2.2. Diffusion Cell Experiments
3. Results
3.1. Membrane Synthesis, Water Uptake, Water Volume Fraction, and Crosslinking Density
3.2. Glass Transition Temperature
3.3. Dimensional Swelling
3.4. Single and Multi-Solute Permeability
3.4.1. Single Solute Permeability
3.4.2. Multi-Solute Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, N.; Suzuki, N.; Terashima, C.; Fujishima, A. Recent Improvements in the Production of Solar Fuels: From CO2 Reduction to Water Splitting and Artificial Photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Altaf, N.; Huang, L.; Gao, Y.; Wang, Q. Electrolytic Cell Design for Electrochemical CO2 Reduction. J. CO2 Util. 2019, 35, 90–105. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef] [PubMed]
- Chabi, S.; Papadantonakis, K.M.; Lewis, N.S.; Freund, M.S. Membranes for Artificial Photosynthesis. Energy Environ. Sci. 2017, 10, 1320–1338. [Google Scholar] [CrossRef]
- Chen, Y.; Lewis, N.S.; Xiang, C. Operational Constraints and Strategies for Systems to Effect the Sustainable, Solar-Driven Reduction of Atmospheric CO2. Energy Environ. Sci. 2015, 8, 3663–3674. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Sulfonated Fluorinated Multi-Block Copolymer Hybrid Containing Sulfonated (Poly Ether Ether Ketone) and Graphene Oxide: A Ternary Hybrid Membrane Architecture for Electrolyte Applications in Proton Exchange Membrane Fuel Cells. J. Energy Chem. 2018, 27, 1247–1260. [Google Scholar] [CrossRef]
- Ion-Ebrasu, D.; Pollet, B.G.; Spinu-Zaulet, A.; Soare, A.; Carcadea, E.; Varlam, M.; Caprarescu, S. Graphene Modified Fluorinated Cation-Exchange Membranes for Proton Exchange Membrane Water Electrolysis. Int. J. Hydrog. Energy 2019, 44, 10190–10196. [Google Scholar] [CrossRef]
- Ion-Ebrasu, D.; Pollet, B.G.; Caprarescu, S.; Chitu, A.; Trusca, R.; Niculescu, V.; Gabor, R.; Carcadea, E.; Varlam, M.; Vasile, B.S. Graphene Inclusion Effect on Anion-Exchange Membranes Properties for Alkaline Water Electrolyzers. Int. J. Hydrog. Energy 2020, 45, 17057–17066. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Grzegorzek, M.; Majewska-Nowak, K.; Ahmed, A.E. Removal of Fluoride from Multicomponent Water Solutions with the Use of Monovalent Selective Ion-Exchange Membranes. Sci. Total Environ. 2020, 722, 137681. [Google Scholar] [CrossRef]
- Crothers, A.R.; Darling, R.M.; Kushner, D.I.; Perry, M.L.; Weber, A.Z. Theory of Multicomponent Phenomena in Cation-Exchange Membranes: Part III. Transport in Vanadium Redox-Flow-Battery Separators. J. Electrochem. Soc. 2020, 167, 013549. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Golubenko, D.V. Membrane Materials for Energy Production and Storage. Pure Appl. Chem. 2020, 92, 1147–1157. [Google Scholar] [CrossRef]
- Singh, M.R.; Bell, A.T. Design of an Artificial Photosynthetic System for Production of Alcohols in High Concentration from CO2. Energy Environ. Sci. 2015, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane Materials for Water Purification: Design, Development, and Application. Environ. Sci. Water Res. Technol. 2015, 2, 17–42. [Google Scholar] [CrossRef]
- Dobyns, B.M.; Kim, J.M.; Beckingham, B.S. Multicomponent Transport of Methanol and Sodium Acetate in Poly (Ethylene Glycol) Diacrylate Membranes of Varied Fractional Free Volume. Eur. Polym. J. 2020, 134, 109809. [Google Scholar] [CrossRef]
- Kim, J.M.; Dobyns, B.M.; Zhao, R.; Beckingham, B.S. Multicomponent Transport of Methanol and Acetate in a Series of Crosslinked PEGDA-AMPS Cation Exchange Membranes. J. Membr. Sci. 2020, 614, 118486. [Google Scholar] [CrossRef]
- Kim, J.M.; Beckingham, B.S. Comonomer Effects on Co-Permeation of Methanol and Acetate in Cation Exchange Membranes. Eur. Polym. J. 2021, 147, 110307. [Google Scholar] [CrossRef]
- Kim, J.M.; Mazumder, A.; Li, J.; Jiang, Z.; Beckingham, B.S. Impact of PEGMA on Transport and Co-Transport of Methanol and Acetate in PEGDA-AMPS Cation Exchange Membranes. J. Membr. Sci. 2022, 642, 119950. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked Poly (Ethylene Glycol) Diacrylate (PEGDA) Hydrogels from Low Molecular Weight Prepolymer: Swelling and Permeation Studies. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ni, L.; Meng, J.; Geise, G.M.; Zhang, Y.; Zhou, J. Water and Salt Transport Properties of Zwitterionic Polymers Film. J. Membr. Sci. 2015, 491, 73–81. [Google Scholar] [CrossRef]
- Yan, N.; Sujanani, R.; Kamcev, J.; Jang, E.-S.; Kobayashi, K.; Paul, D.R.; Freeman, B.D. Salt and Ion Transport in a Series of Crosslinked AMPS/PEGDA Hydrogel Membranes. J. Membr. Sci. 2022, 653, 120549. [Google Scholar] [CrossRef]
- Ju, H.; Sagle, A.C.; Freeman, B.D.; Mardel, J.I.; Hill, A.J. Characterization of Sodium Chloride and Water Transport in Crosslinked Poly (Ethylene Oxide) Hydrogels. J. Membr. Sci. 2010, 358, 131–141. [Google Scholar] [CrossRef]
- Galizia, M.; Paul, D.R.; Freeman, B.D. Liquid Methanol Sorption, Diffusion and Permeation in Charged and Uncharged Polymers. Polymer 2016, 102, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Beckingham, B.S.; Lynd, N.A.; Miller, D.J. Monitoring Multicomponent Transport Using in Situ ATR FTIR Spectroscopy. J. Membr. Sci. 2018, 550, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Kalakkunnath, S.; Kalika, D.S.; Lin, H.; Freeman, B.D. Segmental Relaxation Characteristics of Cross-Linked Poly (Ethylene Oxide) Copolymer Networks. Macromolecules 2005, 38, 9679–9687. [Google Scholar] [CrossRef]
- Tran, T.N.; Ramanan, S.N.; Lin, H. Synthesis of Hydrogels with Antifouling Properties As Membranes for Water Purification. J. Vis. Exp. 2017, 122, e55426. [Google Scholar] [CrossRef]
- Mazumder, A.; Dobyns, B.M.; Howard, M.P.; Beckingham, B.S. Theoretical and Experimental Considerations for Investigating Multicomponent Diffusion in Hydrated, Dense Polymer Membranes. Membranes 2022, 12, 942. [Google Scholar] [CrossRef]
- Gabler, S.; Stampfl, J.; Koch, T.; Seidler, S.; Schuller, G.; Redl, H.; Juras, V.; Trattnig, S.; Weidisch, R. Determination of the Viscoelastic Properties of Hydrogels Based on Polyethylene Glycol Diacrylate (PEG-DA) and Human Articular Cartilage. Int. J. Mater. Eng. Innov. 2009, 1, 3. [Google Scholar] [CrossRef]
- Lin, H.; Wagner, E.V.; Swinnea, J.S.; Freeman, B.D.; Pas, S.J.; Hill, A.J.; Kalakkunnath, S.; Kalika, D.S. Transport and Structural Characteristics of Crosslinked Poly (Ethylene Oxide) Rubbers. J. Membr. Sci. 2006, 276, 145–161. [Google Scholar] [CrossRef]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Kamcev, J.; Freeman, B.D. Charged Polymer Membranes for Environmental/Energy Applications. Annu. Rev. Chem. Biomol. 2015, 7, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Lamaze, C.E.; Ikenberry, L.D. Permeability of solutes through hydrated polymer membranes. Part I. Diffusion of sodium chloride. Die Makromol. Chem. 1968, 118, 19–35. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, Y.; Hunter, B.; Beckingham, B.S. Transport and Co-Transport of Carboxylate Ions and Ethanol in Anion Exchange Membranes. Polymers 2021, 13, 2885. [Google Scholar] [CrossRef]
- Vany’sek, P. Ionic Conductivity and Diffusion at Infinite Dilution. In CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hills, E.E.; Abraham, M.H.; Hersey, A.; Bevan, C.D. Diffusion Coefficients in Ethanol and in Water at 298K: Linear Free Energy Relationships. Fluid Phase Equilibria 2011, 303, 45–55. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Equilibrium ion partitioning between aqueous salt solutions and inhomogeneous ion exchange membranes. Desalination 2018, 446, 31–41. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Ion Activity Coefficients in Ion Exchange Polymers: Applicability of Manning’s Counterion Condensation Theory. Macromolecules 2015, 48, 8011–8024. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Ion Diffusion Coefficients in Ion Exchange Membranes: Significance of Counterion Condensation. Macromolecules 2018, 51, 5519–5529. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazumder, A.; Kim, J.M.; Hunter, B.; Beckingham, B.S. Controlling Fractional Free Volume, Transport, and Co-Transport of Alcohols and Carboxylate Salts in PEGDA Membranes. Membranes 2023, 13, 17. https://doi.org/10.3390/membranes13010017
Mazumder A, Kim JM, Hunter B, Beckingham BS. Controlling Fractional Free Volume, Transport, and Co-Transport of Alcohols and Carboxylate Salts in PEGDA Membranes. Membranes. 2023; 13(1):17. https://doi.org/10.3390/membranes13010017
Chicago/Turabian StyleMazumder, Antara, Jung Min Kim, Brock Hunter, and Bryan S. Beckingham. 2023. "Controlling Fractional Free Volume, Transport, and Co-Transport of Alcohols and Carboxylate Salts in PEGDA Membranes" Membranes 13, no. 1: 17. https://doi.org/10.3390/membranes13010017