Initial Arterial pCO2 and Its Course in the First Hours of Extracorporeal Cardiopulmonary Resuscitation Show No Association with Recovery of Consciousness in Humans: A Single-Centre Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
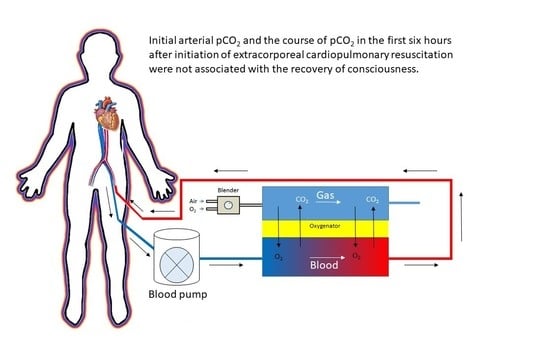
2.2. ECPR Procedure
2.3. Measured Variables
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Laboratory Results
3.3. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statements
References
- Rea, T.D.; Cook, A.J.; Hallstrom, A. CPR during ischemia and reperfusion: A model for survival benefits. Resuscitation 2008, 77, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmerhorst, H.J.; Roos-Blom, M.J.; van Westerloo, D.J.; Abu-Hanna, A.; de Keizer, N.F.; de Jonge, E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit. Care 2015, 19, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, B.W.; Kilgannon, J.H.; Hunter, B.R.; Puskarich, M.A.; Pierce, L.; Donnino, M.; Leary, M.; Kline, J.A.; Jones, A.E.; Shapiro, N.I.; et al. Association between Early Hyperoxia Exposure after Resuscitation from Cardiac Arrest and Neurological Disability: Prospective Multicenter Protocol-Directed Cohort Study. Circulation 2018, 137, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Prince, D.K.; Drennan, I.R.; Grunau, B.; Carlbom, D.J.; Johnson, N.; Hansen, M.; Elmer, J.; Christenson, J.; Kudenchuk, P.; et al. Post-resuscitation arterial oxygen and carbon dioxide and outcomes after out-of-hospital cardiac arrest. Resuscitation 2017, 120, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ebner, F.; Harmon, M.B.; Aneman, A.; Cronberg, T.; Friberg, H.; Hassager, C.; Juffermans, N.; Kjærgaard, J.; Kuiper, M.; Mattsson, N.; et al. Carbon dioxide dynamics in relation to neurological outcome in resuscitated out-of-hospital cardiac arrest patients: An exploratory Target Temperature Management Trial substudy. Crit. Care 2018, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Vaahersalo, J.; Bendel, S.; Reinikainen, M.; Kurola, J.; Tiainen, M.; Raj, R.; Pettilä, V.; Varpula, T.; Skrifvars, M.B.; FINNRESUSCI Study Group. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: Associations with long-term neurologic outcome. Crit. Care Med. 2014, 42, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wang, C.H.; Lai, C.H.; Yu, H.Y.; Chou, N.K.; Wang, C.H.; Huang, S.-C.; Tsai, P.R.; Chou, F.-J.; Tsai, M.-S.; et al. Optimal Arterial Blood Oxygen Tension in the Early Postresuscitation Phase of Extracorporeal Cardiopulmonary Resuscitation: A 15-Year Retrospective Observational Study. Crit. Care Med. 2019, 47, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Halter, M.; Jouffroy, R.; Saade, A.; Philippe, P.; Carli, P.; Vivien, B. Association between hyperoxemia and mortality in patients treated by eCPR after out-of-hospital cardiac arrest. Am. J. Emerg. Med. 2020, 38, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Cavayas, Y.A.; Munshi, L.; Del Sorbo, L.; Fan, E. The Early Change in PaCO2 after Extracorporeal Membrane Oxygenation Initiation Is Associated with Neurological Complications. Am. J. Respir. Crit. Care Med. 2020, 201, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Berg, R.A.; Andersen, L.W.; Bhanji, F.; Chan, P.S.; Donnino, M.W.; Lim, S.H.; Ma, M.H.-M.; Nadkarni, V.M.; Starks, M.A.; et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: A Consensus Report From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Circulation 2019, 140, e746–e757. [Google Scholar] [PubMed] [Green Version]
- Bemtgen, X.; Schroth, F.; Wengenmayer, T.; Biever, P.M.; Duerschmied, D.; Benk, C.; Bode, C.; Staudacher, D.L. How to treat combined respiratory and metabolic acidosis after extracorporeal cardiopulmonary resuscitation? Crit. Care 2019, 23, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartos, J.A.; Grunau, B.; Carlson, C.; Duval, S.; Ripeckyj, A.; Kalra, R.; Raveendran, G.; John, R.; Conterato, M.; Frascone, R.J.; et al. Improved Survival with Extracorporeal Cardiopulmonary Resuscitation Despite Progressive Metabolic Derangement Associated with Prolonged Resuscitation. Circulation 2020, 141, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Umemoto, N.; Taniguchi, T.; Ishii, H.; Hiramatsu, Y.; Arata, K.; Takuya, H.; Inoue, S.; Sugiura, T.; Asai, T.; et al. The lactate clearance calculated using serum lactate level 6 h after is an important prognostic predictor after extracorporeal cardiopulmonary resuscitation: A single-center retrospective observational study. J. Intensive Care 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.T.; Beesems, S.G.; Homma, P.C.M.; Zijlstra, J.A.; Hulleman, M.; Van Hoeijen, D.A.; Bardai, A.; Tijssen, J.G.P.; Tan, H.L.; Koster, R.W. Improved Survival After Out-of-Hospital Cardiac Arrest and Use of Automated External Defibrillators. Circulation 2014, 130, 1868–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartstichting. Jaarverslag 2019. Werken aan een Hartgezonde Samenleving. Available online: https://magazines.hartstichting.nl/jaarverslag2019/cover/?_ga=2.261012493.1236433838.1615061661-1639466317.1605858018 (accessed on 5 March 2021).
| Total | Recovery of Consciousness (N = 32) | No Recovery of Consciousness (N = 56) | p-Value | |
|---|---|---|---|---|
| Patient number (%) | 84 | 32 (38.1) | 52 (61.9) | |
| Patient characteristics | ||||
| Age in years (SD) | 46.9 (15.6) | 47.1 (15.5) | 46.7 (15.7) | 0.91 |
| Sex; Male (%) | 53 (63.1) | 19 (59.4) | 34 (65.4) | 0.75 |
| BMI (IQR) | 26.3 (24.6–29.8) | 26.5 (25.2–30.1) | 25.8 (24.5–29.4) | 0.40 |
| Clinical characteristics | ||||
| APACHE IV-score (SD) (Missing N = 36) | 112 (31) | 110 (36) | 113 (27) | 0.74 |
| Witnessed arrest (%) | 78 (92.9) | 31 (96.9) | 47 (90.4) | 0.40 |
| OHCA (%) | 38 (45.2) | 13 (40.6) | 25 (48.1) | 0.66 |
| BLS (%) | 37 (44.0) | 15 (46.9) | 22 (42.3) | 0.85 |
| Direct life support (%) | 79 (94.0) | 30 (93.8) | 49 (94.2) | 1.00 |
| No-flow in minutes (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.25 |
| Total low-flow duration in minutes (IQR) (Missing N = 3) | 51.0 (37.0–80.0) | 45.0 (30.0–76.5) | 58.0 (40.0–84.0) | 0.24 |
| Mechanical compression device, e.g., LUCAS (%) | 27 (32.1) | 6 (18.8) | 21 (41.2) | 0.06 |
| Primary cardiac rhythm | ||||
| Shockable (%) | 26 (31.0) | 11 (34.4) | 15 (29.4) | 0.82 |
| Ventricular fibrillation (%) | 23 (27.4) | 9 (28.0) | 14 (28.1) | 1.00 |
| Ventricular tachycardia (%) | 3 (3.6) | 2 (6.3) | 1 (2.0) | 0.56 |
| Non-shockable (%) | 57 (68.7) | 21 (65.6) | 36 (70.6) | 0.82 |
| Pulseless electrical activity (%) | 47 (56.0) | 20 (62.5) | 27 (54.0) | 0.60 |
| Asystole (%) | 9 (10.7) | 1 (3.1) | 8 (16.0) | 0.08 |
| Location of arrest | ||||
| Home (%) | 23 (27.4) | 8 (25.0) | 15 (28.8) | 0.90 |
| Public (%) | 13 (15.5) | 5 (15.6) | 8 (15.4) | 1.00 |
| ICU (%) | 24 (28.6) | 9 (28.1) | 16 (30.8) | 0.99 |
| Ward (%) | 10 (11.9) | 3 (9.4) | 7 (13.5) | 0.73 |
| Emergency department (%) | 4 (4.8) | 1 (3.1) | 3 (5.8) | 1.00 |
| Operation room (%) | 4 (4.8) | 3 (9.4) | 1 (1.9) | 0.15 |
| Catherisation laboratory (%) | 3 (3.6) | 3 (9.4) | 0 (0.0) | 0.14 |
| Other (%) | 1 (1.2) | 0 (0.0) | 1 (1.9) | 1.00 |
| Cause of arrest | ||||
| Acute coronary syndrome (%) | 25 (29.8) | 12 (37.5) | 13 (25.0) | 0.33 |
| Pulmonary embolism (%) | 30 (35.7) | 11 (34.4) | 19 (36.5) | 1.00 |
| Tamponade (%) | 3 (3.6) | 2 (6.3) | 1 (1.9) | 0.55 |
| Hypothermia/drowning (%) | 5 (6.0) | 1 (3.1) | 4 (7.8) | 0.64 |
| Post cardiac surgery (%) | 2 (2.4) | 0 (0.0) | 2 (3.8) | 0.52 |
| Myocarditis (%) | 3 (3.6) | 2 (6.3) | 1 (1.9) | 0.55 |
| Heart failure (%) | 3 (3.6) | 2 (6.3) | 1 (1.9) | 0.55 |
| Hypoxemia (%) | 2 (2.4) | 0 (0.0) | 2 (3.8) | 0.52 |
| Sepsis (%) | 2 (2.4) | 1 (3.1) | 1 (1.9) | 1.00 |
| Other (%) | 7 (8.3) | 0 (0.0) | 7 (12.5) | 0.04 |
| Unknown (%) | 2 (2.4) | 1 (3.1) | 1 (1.9) | 1.00 |
| Complications | ||||
| Bleeding (%) | 56 (66.7) | 24 (75) | 32 (61.5) | 0.30 |
| Limb ischaemia (%) | 5 (6.0) | 2 (6.3) | 3 (5.8) | 1.00 |
| Cerebrovascular accident (%) | 6 (7.1) | 4 (12.5) | 2 (3.8) | |
| Cerebral bleeding (%) | 5 (6.0) | 3 (9.4) | 2 (3.8) | 0.36 |
| Cerebral infarction (%) | 1 (1.2) | 1 (3.1) | 0 (0.0) | 0.38 |
| Infection (%) | 28 (33.3) | 16 (50.0) | 12 (25.0) | 0.04 |
| Acute kidney injury (%) | 43 (51.2) | 21 (65.6) | 23 (44.2) | 0.09 |
| CRRT (%) | 15 (17.9) | 6 (18.8) | 9 (17.3) | 1.00 |
| Tamponade (%) | 6 (7.1) | 2 (6.3) | 4 (7.7) | 1.00 |
| Abdominal compartment syndrome (%) | 4 (4.8) | 1 (3.1) | 3 (5.8) | 1.00 |
| Laboratory results | ||||
| Initial pCO2 in kPa (IQR) | 7.3 (5.7–9.9) | 7.1 (5.3–8.9) | 7.7 (6.0–9.9) | 0.30 |
| Course of pCO2 in %/h (IQR) | −5.22 (−8.69 to −1.99) | −4.09 (−8.38 to −1.30) | −6.28 (−8.69 to −2.08) | 0.37 |
| Maximum decrease pCO2 in %/hour (IQR) | 0.67 (0.38–1.06) | 0.58 (0.24–1.06) | 0.72 (0.41–0.82) | 0.43 |
| Maximum difference pCO2 in %/hour (IQR) | −0.52 (−0.87 to 0.39) | −0.30 (−0.88 to 0.08) | −0.59 (−0.86 to 0.71) | 0.76 |
| Initial pO2 in kPa (IQR) | 25.3 (10.8–43.5) | 17.4 (9.4–42.5) | 32.8 (11.5–47.1) | 0.10 |
| Course of pO2 in %/hour (IQR) | −6.29 (−11.65 to 9.31) | −4.26 (−12.85 to 12.53) | −7.15 (−11.30 to 5.97) | 0.90 |
| Initial pH (IQR) | 6.96 (6.80–7.08) | 7.07 (6.84–7.21) | 6.90 (6.79–7.00) | <0.01 |
| Course of pH in %/hour (SD) | 0.68 (0.53) | 0.68 (0.48) | 0.69 (0.57) | 0.98 |
| Initial lactate in mmol/L (SD) | 13.7 (5.8) | 12.5 (6.0) | 14.5 (5.7) | 0.14 |
| Course of lactate in %/h (IQR) | −7.44 (−11.89 to −1.33) | −10.38 (−12.98 to −5.06) | −6.11 (−11.12 to −5.48) | <0.05 |
| Outcomes | ||||
| ECMO survival (%) | 32 (38.1) | 28 (87.5) | 4 (7.7) | <0.01 |
| ICU-survival (%) | 25 (29.8) | 24 (75.0) | 1 (1.9) | <0.01 |
| Hospital survival (%) | 24 (28.6) | 23 (71.9) | 1 (1.9) | |
| Cause of death | N = 59 | N = 9 | N = 50 | |
| Brain death (%) | 5 (8.5) | 0 (0.0) | 5 (10.0) | 1.00 |
| Neurology (%) | 23 (39.0) | 4 (44.4) | 19 (38.0) | 0.73 |
| Cardiac (%) | 4 (6.8) | 1 (11.1) | 3 (6.0) | 0.49 |
| Haemorrhagic shock (%) | 2 (3.4) | 0 (0.0) | 2 (4.0) | 1.00 |
| Multi-organ disease (%) | 14 (23.7) | 2 (22.2) | 13 (26.0) | 1.00 |
| Persisting cardiac arrest (%) | 2 (3.4) | 0 (0.0) | 2 (4.0) | 1.00 |
| Other (%) | 7 (11.9) | 2 (22.2) | 5 (10.0) | 0.29 |
| (a) | (b) | (c) | (d) | (e) | |
|---|---|---|---|---|---|
| Initial pCO2 | 0.93 (0.78–1.09) | 0.97 (0.79–1.20) | 0.92 (0.65–1.30) | 0.94 (0.78–1.12) | 0.75 (0.52–1.05) |
| Course of pCO2 in first 6 h | 1.03 (0.9–1.13) | 1.05 (0.92–1.26) | |||
| Interaction initial and course pCO2 | 0.99 (0.97–1.02) | ||||
| Maximum decrease of pCO2 in first 6 h | 1.07 (0.48–2.30) | 0.14 (0.01–2.07) | |||
| Interaction initial and maximum decrease pCO2 | 1.29 (0.93–1.88) | ||||
| N | 83 | 83 | 83 | 80 | 80 |
| Nagelkerke R2 | 0.03 | 0.04 | 0.04 | 0.10 | 0.13 |
| AIC | 113.82 | 115.22 | 117.06 | 111.36 | 111.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandigers, L.; den Uil, C.A.; Bunge, J.J.H.; Gommers, D.; dos Reis Miranda, D. Initial Arterial pCO2 and Its Course in the First Hours of Extracorporeal Cardiopulmonary Resuscitation Show No Association with Recovery of Consciousness in Humans: A Single-Centre Retrospective Study. Membranes 2021, 11, 208. https://doi.org/10.3390/membranes11030208
Mandigers L, den Uil CA, Bunge JJH, Gommers D, dos Reis Miranda D. Initial Arterial pCO2 and Its Course in the First Hours of Extracorporeal Cardiopulmonary Resuscitation Show No Association with Recovery of Consciousness in Humans: A Single-Centre Retrospective Study. Membranes. 2021; 11(3):208. https://doi.org/10.3390/membranes11030208
Chicago/Turabian StyleMandigers, Loes, Corstiaan A. den Uil, Jeroen J. H. Bunge, Diederik Gommers, and Dinis dos Reis Miranda. 2021. "Initial Arterial pCO2 and Its Course in the First Hours of Extracorporeal Cardiopulmonary Resuscitation Show No Association with Recovery of Consciousness in Humans: A Single-Centre Retrospective Study" Membranes 11, no. 3: 208. https://doi.org/10.3390/membranes11030208