Anion Exchange Membranes Prepared from Quaternized Polyepichlorohydrin Cross-Linked with 1-(3-aminopropyl)imidazole Grafted Poly(arylene ether ketone) for Enhancement of Toughness and Conductivity
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Anion Exchange Membrane
2.2.1. Synthesis of PAEK-API Precursors
2.2.2. Preparation of QPECH/PAEK-API Membranes
2.3. Characterization
2.3.1. Chemical Structure Analysis
2.3.2. Ion Exchange Capacity (IEC)
2.3.3. Anion Conductivity
2.3.4. Water Uptake and Length Swelling Percentage
2.3.5. Ion Cluster Dimension
2.3.6. Thermal, Mechanical, and Chemical Stability
3. Results and Discussion
3.1. Chemical Structure Analysis
3.2. Ion Exchange Capacity (IEC)
3.3. Water Uptake and Length Swelling Percentage
3.4. Anion Conductivity
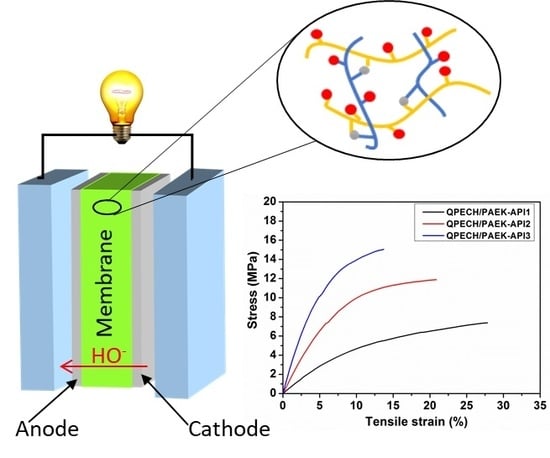
3.5. Mechanical Property
3.6. Thermal Property
3.7. Ion Cluster Structure
3.8. Chemical Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hunger, H. Ion-exchange liquid-fuel cells. In Proceedings of the Annual Power Sources Conference, 1960 U.S. Army Signal REsearch and Development Laboratory, Power Sources Division, Atlantic City, NJ, USA, 17–19 May 1960; pp. 55–59. [Google Scholar]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Vo, D.C.T.; Nguyen, M.D.T.; Kim, D. Dual sulfonated poly(arylene ether ketone) membrane grafted with 15-crown-5-ether for enhanced proton conductivity and anti-oxidation stability. Mol. Syst. Des. Eng. 2019, 4, 901–911. [Google Scholar] [CrossRef]
- Nguyen, M.D.T.; Yang, S.; Kim, D. Pendant dual sulfonated poly(arylene ether ketone) proton exchange membranes for fuel cell application. J. Power Sources 2016, 328, 355–363. [Google Scholar] [CrossRef]
- Iojoiu, C.; Chabert, F.; Maréchal, M.; Kissi, N.; Guindet, J.; Sanchez, J.Y. From polymer chemistry to membrane elaboration. J. Power Sources 2006, 153, 198–209. [Google Scholar] [CrossRef]
- Tham, D.D.; Kim, D. C2 and N3 substituted imidazolium functionalized poly(arylene ether ketone) anion exchange membrane for water electrolysis with improved chemical stability. J. Membr. Sci. 2019, 581, 139–149. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.; Varcoe, J.R. Comparison of PVDF- and FEP-based radiation-grafted alkaline anion-exchange membranes for use in low temperature portable DMFCs. J. Mater. Chem. 2002, 12, 3371–3373. [Google Scholar] [CrossRef] [Green Version]
- ZOgumi, Z.; Matsuoka, K.; Chiba, S.; Matsuoka, M.; Iriyama, Y.; Abe, T.; Inaba, M. Preliminary Study on Direct Alcohol Fuel Cells Employing Anion Exchange Membrane. Electrochem. 2002, 70, 980–983. [Google Scholar]
- Danks, T.N.; Slade, R.; Varcoe, J.R. Alkaline anion-exchange radiation-grafted membranes for possible electrochemical application in fuel cells. J. Mater. Chem. 2003, 13, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.; Shukla, A.; Jackson, C.; Meuleman, W. A mixed-reactants solid-polymer-electrolyte direct methanol fuel cell. J. Power Sources 2004, 126, 67–75. [Google Scholar] [CrossRef]
- Rahim, M.A.; Hameed, R.A.; Khalil, M. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Tripković, A.V.; Popović, K.D.; Grgur, B.N.; Blizanac, B.; Ross, P.; Marković, N. Methanol electrooxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim. Acta 2002, 47, 3707–3714. [Google Scholar] [CrossRef]
- Asazawa, K.; Yamada, K.; Tanaka, H.; Oka, A.; Taniguchi, M.; Kobayashi, T. A platinum-free zero-carbon-emission easy fuelling direct hydrazine fuel cell for vehicles. Angew. Chem. Int. Ed. 2007, 46, 8024–8027. [Google Scholar] [CrossRef] [PubMed]
- Lamy, C.; Belgsir, E.; Leger, J. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC). J. Appl. Electrochem. 2001, 31, 799–809. [Google Scholar] [CrossRef]
- Manoharan, R.; Prabhuram, J. Possibilities of prevention of formation of poisoning species on direct methanol fuel cell anodes. J. Power Sources 2001, 96, 220–225. [Google Scholar] [CrossRef]
- Yu, E.; Scott, K.; Reeve, R. Electrochemical reduction of oxygen on carbon supported Pt and Pt/Ru fuel cell electrodes in alkaline solutions. Fuel Cells 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Tuan, C.M.; Patra, A.K.; Kim, D. Chemically modified poly (arylene ether ketone) s with pendant imidazolium groups: Anion exchange membranes for alkaline fuel cells. Int. J. Hydrog. Energy 2018, 43, 4517–4527. [Google Scholar] [CrossRef]
- Yu, E.; Scott, K. Direct methanol alkaline fuel cells with catalysed anion exchange membrane electrodes. J. Appl. Electrochem. 2005, 35, 91–96. [Google Scholar] [CrossRef]
- Zarrin, H.; Wu, J.; Fowler, M.; Chen, Z.J.J.o.m.s. High durable PEK-based anion exchange membrane for elevated temperature alkaline fuel cells. J. Membr. Sci. 2012, 394, 193–201. [Google Scholar] [CrossRef]
- Liu, G.; Shang, Y.; Xie, X.; Wang, S.; Wang, J.; Wang, Y.; Mao, Z. Synthesis and characterization of anion exchange membranes for alkaline direct methanol fuel cells. Int. J. Hydrog. Energy 2012, 37, 848–853. [Google Scholar] [CrossRef]
- Guo, M.; Fang, J.; Xu, H.; Li, W.; Lu, X.; Lan, C.; Li, K. Synthesis and characterization of novel anion exchange membranes based on imidazolium-type ionic liquid for alkaline fuel cells. J. Membr. Sci. 2010, 362, 97–104. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Kim, M.-K.; Yun, S.-H.; Moon, S.-H. Stability of composite anion exchange membranes with various functional groups and their performance for energy conversion. J. Membr. Sci. 2013, 443, 28–35. [Google Scholar] [CrossRef]
- Wang, G.; Weng, Y.; Chu, D.; Xie, D.; Chen, R. Preparation of alkaline anion exchange membranes based on functional poly (ether-imide) polymers for potential fuel cell applications. J. Membr. Sci. 2009, 326, 4–8. [Google Scholar] [CrossRef]
- Guo, T.Y.; Zeng, Q.H.; Zhao, C.H.; Liu, Q.L.; Zhu, A.M.; Broadwell, I. Quaternized polyepichlorohydrin/PTFE composite anion exchange membranes for direct methanol alkaline fuel cells. J. Membr. Sci. 2011, 371, 268–275. [Google Scholar] [CrossRef]
- Tuan, C.M.; Kim, D. Anion-exchange membranes based on poly (arylene ether ketone) with pendant quaternary ammonium groups for alkaline fuel cell application. J. Membr. Sci. 2016, 511, 143–150. [Google Scholar] [CrossRef]










| Samples | IEC (mequiv g−1) | Mechanical property | WU (%) | SR (%) | ET (J mol−1 K−1) | |
|---|---|---|---|---|---|---|
| Stress (MPa) | Elongation (%) | |||||
| QPECH/PAEK-API1 | 1.25 | 15.07 | 14.52 | 39.72 | 9.38 | 7.468 |
| QPECH/PAEK-API2 | 0.97 | 12.01 | 20.23 | 27.43 | 8.43 | 11.202 |
| QPECH/PAEK-API3 | 0.78 | 7.32 | 27.92 | 23.27 | 7.34 | 16.239 |
| AHA | - | - | - | - | - | 34.203 |
| Temperature (°C) | QPECH/PAEK-API1 | QPECH/PAEK-API2 | QPECH/PAEK-API3 | AHA Membranes | ||||
|---|---|---|---|---|---|---|---|---|
| Zre | −Zim | Zre | −Zim | Zre | −Zim | Zre | −Zim | |
| 30 | 2.409 | 0.446 | 5.777 | 1.236 | 39.853 | 10.428 | 11.463 | 2.710 |
| 40 | 2.110 | 0.410 | 5.085 | 1.101 | 27.611 | 7.204 | 9.211 | 2.200 |
| 50 | 1.898 | 0.376 | 4.487 | 0.950 | 17.580 | 4.562 | 7.769 | 1.806 |
| 60 | 1.748 | 0.353 | 3.894 | 0.756 | 11.165 | 2.631 | 6.375 | 1.507 |
| 70 | 1.654 | 0.280 | 3.442 | 0.702 | 7.821 | 1.976 | 5.329 | 1.260 |
| 80 | 1.604 | 0.178 | 3.117 | 0.687 | 6.547 | 1.512 | 4.648 | 0.986 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuan, C.M.; Cong Tinh, V.D.; Kim, D. Anion Exchange Membranes Prepared from Quaternized Polyepichlorohydrin Cross-Linked with 1-(3-aminopropyl)imidazole Grafted Poly(arylene ether ketone) for Enhancement of Toughness and Conductivity. Membranes 2020, 10, 138. https://doi.org/10.3390/membranes10070138
Tuan CM, Cong Tinh VD, Kim D. Anion Exchange Membranes Prepared from Quaternized Polyepichlorohydrin Cross-Linked with 1-(3-aminopropyl)imidazole Grafted Poly(arylene ether ketone) for Enhancement of Toughness and Conductivity. Membranes. 2020; 10(7):138. https://doi.org/10.3390/membranes10070138
Chicago/Turabian StyleTuan, Cao Manh, Vo Dinh Cong Tinh, and Dukjoon Kim. 2020. "Anion Exchange Membranes Prepared from Quaternized Polyepichlorohydrin Cross-Linked with 1-(3-aminopropyl)imidazole Grafted Poly(arylene ether ketone) for Enhancement of Toughness and Conductivity" Membranes 10, no. 7: 138. https://doi.org/10.3390/membranes10070138