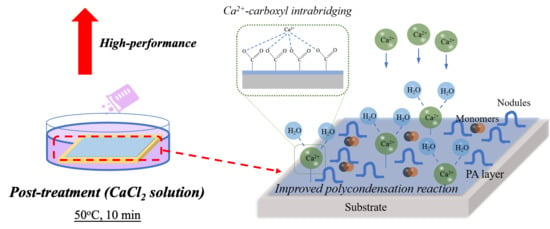
Fabrication of High-Performance Thin-Film Composite Nanofiltration Membrane by Dynamic Calcium-Carboxyl Intra-Bridging during Post-Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Nanofiltration Performance Tests
3. Results and Discussion
3.1. Membrane Surface Morphology
3.2. Chemical Composition of Polyamide Layer
3.3. Surface Charge and Hydrophilicity
3.4. Mechanisms of Dynamic Modification Method
3.5. Separation Performance of the Composite NF Membranes
3.6. Antifouling Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef] [Green Version]
- Kummu, M.; Ward, P.; De Moel, H.; Varis, O. Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ. Res. Lett. 2010, 5, 034006. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Asefa, T.; Bracciano, D.; Adams, A.; Wanakule, N. Proactive water shortage mitigation integrating system optimization and input uncertainty. J. Hydrol. 2019, 571, 711–722. [Google Scholar] [CrossRef]
- Li, X.; Jiang, W.; Duan, D. Spatio-temporal analysis of irrigation water use coefficients in China. J. Environ. Manag. 2020, 262, 110242. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.L.; Suk, D.E.; Nguyen, T.-V.; Hoek, E.M.V. Tailoring the Structure of Thin Film Nanocomposite Membranes to Achieve Seawater RO Membrane Performance. Environ. Sci. Technol. 2010, 44, 8230–8235. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Seeram, R. New directions in nanofiltration applications — Are nanofibers the right materials as membranes in desalination? Desalination 2013, 308, 198–208. [Google Scholar] [CrossRef]
- Fang, W.; Shi, L.; Wang, R. Interfacially polymerized composite nanofiltration hollow fiber membranes for low-pressure water softening. J. Membr. Sci. 2013, 430, 129–139. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Lebrun, R.E.; Xu, Y. Dynamic Characterization of Nanofiltration and Reverse Osmosis Membranes. Sep. Sci. Technol. 1999, 34, 1629–1641. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.; Misdan, N.; Kassim, M. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; McCutcheon, J.R. Understanding the influence of solvents on the intrinsic properties and performance of polyamide thin film composite membranes. Sep. Purif. Technol. 2020, 238, 116398. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Huang, Y.; Yu, S.; Lawless, D.; Feng, X. Thin film composite nanofiltration membranes assembled layer-by-layer via interfacial polymerization from polyethylenimine and trimesoyl chloride. J. Membr. Sci. 2014, 472, 141–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Peng, J.; Zhao, X.; Liu, J.; Zhao, J.; Jiang, Z. Composite nanofiltration membranes prepared by interfacial polymerization with natural material tannic acid and trimesoyl chloride. J. Membr. Sci. 2013, 429, 235–242. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.; Rahimpour, A. Fabrication and development of interfacial polymerized thin-film composite nanofiltration membrane using different surfactants in organic phase; study of morphology and performance. J. Membr. Sci. 2009, 343, 219–228. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Zhao, P.; Liang, H.; Zhang, W.; Crittenden, J. High-performance polyamide thin-film composite nanofiltration membrane: Role of thermal treatment. Appl. Surf. Sci. 2018, 435, 415–423. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.; Rahimpour, A.; Farhadian, A. The effect of non-contact heating (microwave irradiation) and contact heating (annealing process) on properties and performance of polyethersulfone nanofiltration membranes. Appl. Surf. Sci. 2009, 255, 8395–8402. [Google Scholar] [CrossRef]
- Shi, M.; Yan, W.; Dong, C.; Liu, L.; Xie, S.; Gao, C. Solvent activation before heat-treatment for improving reverse osmosis membrane performance. J. Membr. Sci. 2020, 595, 117565. [Google Scholar] [CrossRef]
- Solomon, M.F.J.; Bhole, Y.; Livingston, A.G. High flux membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization with solvent activation. J. Membr. Sci. 2012, 423, 371–382. [Google Scholar] [CrossRef]
- Han, R.; Zhang, S.; Hu, L.; Guan, S.; Jian, X. Preparation and characterization of thermally stable poly(piperazine amide)/PPBES composite nanofiltration membrane. J. Membr. Sci. 2011, 370, 91–96. [Google Scholar] [CrossRef]
- Hao, X.; Gao, S.; Tian, J.; Wang, S.; Zhang, H.; Sun, Y.; Shi, W.; Cui, F. New insights into the organic fouling mechanism of an in situ Ca2+ modified thin film composite forward osmosis membrane. RSC Adv. 2019, 9, 38227–38234. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Gao, S.; Tian, J.; Sun, Y.; Cui, F.; Tang, C.Y. Calcium-Carboxyl Intrabridging during Interfacial Polymerization: A Novel Strategy to Improve Antifouling Performance of Thin Film Composite Membranes. Environ. Sci. Technol. 2019, 53, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Guo, H.; Tang, C.Y.; Chen, M.; Li, J.; Wang, Z. Hydrophilic Selective Nanochannels Created by Metal Organic Frameworks in Nanofiltration Membranes Enhance Rejection of Hydrophobic Endocrine-Disrupting Compounds. Environ. Sci. Technol. 2019, 53, 13776–13783. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, F.; Ji, Y.; Chen, H. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Ji, Y.-L.; Huang, S.-H.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Incorporation of carboxylic monoamines into thin-film composite polyamide membranes to enhance nanofiltration performance. J. Membr. Sci. 2017, 539, 52–64. [Google Scholar] [CrossRef]
- Fan, X.; Dong, Y.; Su, Y.; Zhao, X.; Li, Y.; Liu, J.; Jiang, Z. Improved performance of composite nanofiltration membranes by adding calcium chloride in aqueous phase during interfacial polymerization process. J. Membr. Sci. 2014, 452, 90–96. [Google Scholar] [CrossRef]
- Zhan, Z.-M.; Xu, Z.-L.; Zhu, K.-K.; Tang, Y.-J. How to understand the effects of heat curing conditions on the morphology and performance of polypiperazine-amide NF membrane. J. Membr. Sci. 2020, 597, 117640. [Google Scholar] [CrossRef]
- Song, X.; Gan, B.; Yang, Z.; Tang, C.Y.; Gao, C. Confined nanobubbles shape the surface roughness structures of thin film composite polyamide desalination membranes. J. Membr. Sci. 2019, 582, 342–349. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Porous metal organic framework CuBDC nanosheet incorporated thin-film nanocomposite membrane for high-performance forward osmosis. J. Membr. Sci. 2019, 573, 46–54. [Google Scholar] [CrossRef]
- Kwon, Y.-N.; Hong, S.; Choi, H.; Tak, T. Surface modification of a polyamide reverse osmosis membrane for chlorine resistance improvement. J. Membr. Sci. 2012, 415, 192–198. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Turturro, A.; Russo, S.; Antolinit, E.; Cirafici, S. Physical properties of anionic poly(e- caprolactam) synthesized in the presence of calcium chloride. Polymer 1989, 30, 1099–1104. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, H.; Li, X.; Liu, Z.; Jia, Q. Study on dry spinning and structure of low mole ratio complex of calcium chloride-polyamide 6. J. Appl. Polym. Sci. 2010. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhao, Y.-Y.; Wang, X.-M.; Wen, X.-H.; Huang, X.; Xie, Y.F. Effect of varying piperazine concentration and post-modification on prepared nanofiltration membranes in selectively rejecting organic micropollutants and salts. J. Membr. Sci. 2019, 582, 274–283. [Google Scholar] [CrossRef]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-template-assisted synthesis of hollow fullerene-like MoS2 nanocages as a lithium ion battery anode material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Hsub, Y.-Z.; Ruaan, R.-C.; Chuang, C.-J.; Tung, K.-L. Nanofiltration membranes synthesized from hyperbranched polyethyleneimine. J. Membr. Sci. 2009, 326, 19–26. [Google Scholar] [CrossRef]
- Xue, S.; Xu, Z.-L.; Tang, Y.-J.; Ji, C.-H. Polypiperazine-amide Nanofiltration Membrane Modified by Different Functionalized Multiwalled Carbon Nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2016, 8, 19135–19144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, G.; Yu, H.; Jin, Y.; Cao, Y. Fabrication of a highly permeable composite nanofiltration membrane via interfacial polymerization by adding a novel acyl chloride monomer with an anhydride group. J. Membr. Sci. 2019, 570–571, 403–409. [Google Scholar] [CrossRef]
- Jun, B.-M.; Yoon, Y.; Park, C.M. Post-Treatment of Nanofiltration Polyamide Membrane through Alkali-Catalyzed Hydrolysis to Treat Dyes in Model Wastewater. Water 2019, 11, 1645. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Barhate, R.; Sundarrajan, S.; Matsuura, T.; Ramakrishna, S. Hot pressing of electrospun membrane composite and its influence on separation performance on thin film composite nanofiltration membrane. Desalination 2011, 279, 201–209. [Google Scholar] [CrossRef]
- Lu, X.; Chavez, L.H.A.; Castrillón, S.R.-V.; Ma, J.; Elimelech, M. Influence of Active Layer and Support Layer Surface Structures on Organic Fouling Propensity of Thin-Film Composite Forward Osmosis Membranes. Environ. Sci. Technol. 2015, 49, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Castrillón, S.R.-V.; Shaffer, D.L.; Ma, J.; Elimelech, M. In Situ Surface Chemical Modification of Thin-Film Composite Forward Osmosis Membranes for Enhanced Organic Fouling Resistance. Environ. Sci. Technol. 2013, 47, 12219–12228. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.; Wu, W.; Docoslis, A.; Giese, R. The interfacial tensions with water and the Lewis acid–base surface tension parameters of polar organic liquids derived from their aqueous solubilities. Colloids Surf. B Biointerfaces 2001, 20, 87–91. [Google Scholar] [CrossRef]
- SkjLk-Brek, G.; Grasdalen, H.; Smidsrod, O. lnhomogeneous Polysaccharide Ionic Gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]










| Sample | Element Content (%) | |||
|---|---|---|---|---|
| C | N | O | Ca | |
| NF-control | 76.86 | 8.51 | 13.81 | 0.45 |
| NF-10 | 71.16 | 6.79 | 15.96 | 2.83 |
| NF-20 | 67.74 | 5.16 | 15.3 | 4.52 |
| NF-40 | 67.1 | 6.35 | 15.86 | 4.53 |
| NF-80 | 69.14 | 5.53 | 14.65 | 4.04 |
| Membrane | Operating Pressure (bar) | Post-Treatment | Water Permeability (L·m−2·h−1·bar−1) | Na2SO4 Rejection (%) | References |
|---|---|---|---|---|---|
| TFC-M3 | 13.8 | thermal treatment | 13.6 | 97.7 | [17] |
| PA@A-0 | 6 | heat curing | 16.7 | 97.4 | [28] |
| PA-16 | 6 | organic solution | 7.6 | 94.9 | [25] |
| PEI/TMC | 4 | ethanol | 9.5 | 56.0 | [38] |
| MWCNT-OH | 6 | heat-treatment | 6.9 | 97.6 | [39] |
| NFM-5 | 6 | heat-treatment | 15.2 | 97.0 | [40] |
| NF-90 | 5 | alkali solution | 15.8 | - | [41] |
| TFNC-2 | 13 | hot pressing | 22.3 | 92.0 | [42] |
| NF-40 | 8 | adding Ca2+ | 29.76 | 98.1 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Dai, R.; Wang, Z. Fabrication of High-Performance Thin-Film Composite Nanofiltration Membrane by Dynamic Calcium-Carboxyl Intra-Bridging during Post-Treatment. Membranes 2020, 10, 137. https://doi.org/10.3390/membranes10070137
Han H, Dai R, Wang Z. Fabrication of High-Performance Thin-Film Composite Nanofiltration Membrane by Dynamic Calcium-Carboxyl Intra-Bridging during Post-Treatment. Membranes. 2020; 10(7):137. https://doi.org/10.3390/membranes10070137
Chicago/Turabian StyleHan, Hongyi, Ruobin Dai, and Zhiwei Wang. 2020. "Fabrication of High-Performance Thin-Film Composite Nanofiltration Membrane by Dynamic Calcium-Carboxyl Intra-Bridging during Post-Treatment" Membranes 10, no. 7: 137. https://doi.org/10.3390/membranes10070137