Behaviour of Aquaporin Forward Osmosis Flat Sheet Membranes during the Concentration of Calcium-Containing Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane and Chemicals
2.2. FO Experiment
2.3. Membrane Characterisation Methods
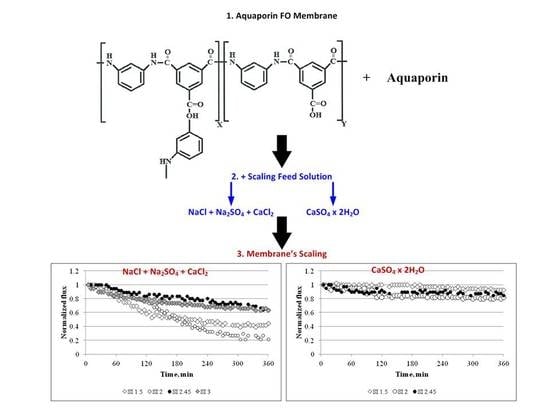
3. Results and Discussion
3.1. Performance of the Membrane without Scalants
3.2. Membrane Behaviour in the Presence of Scaling Solutions
3.3. Characterisation of the Pristine and Scaled Membranes
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E.; Zorpas, A.A.; Menegaki, A.N. Aquatic Environment; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780444627339. [Google Scholar]
- Chung, T.S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO). Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chung, T.S.; Tong, Y.W.; Jeyaseelan, K.; Armugam, A.; Chen, Z.; Hong, M.; Meier, W. Highly permeable and selective pore-spanning biomimetic membrane embedded with aquaporin Z. Small 2012, 8, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.H. Biomimetic membranes for sensor and separation applications. Anal. Bioanal. Chem. 2009, 395, 697–718. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, S.; Stibius, K.; Szewczykowski, P.; Perry, M.; Bohr, H.; Hélix-Nielsen, C. Hydrogels for in situ encapsulation of biomimetic membrane arrays. Polym. Adv. Technol. 2012, 23, 182–189. [Google Scholar] [CrossRef] [Green Version]
- González-Pérez, A.; Stibius, K.B.; Vissing, T.; Nielsen, C.H.; Mouritsen, O.G. Biomimetic triblock copolymer membrane arrays: A stable template for functional membrane proteins. Langmuir 2009, 25, 10447–10450. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Perry, M.; Vogel, J.; Vissing, T.; Hansen, C.R.; Geschke, O.; Emnéus, J.; Nielsen, C.H. Development of an automation technique for the establishment of functional lipid bilayer arrays. J. Micromech. Microeng. 2009, 19, 25014. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chung, T.S.; Tong, Y.W.; Meier, W.; Chen, Z.; Hong, M.; Jeyaseelan, K.; Armugam, A. Preparation and characterization of pore-suspending biomimetic membranes embedded with Aquaporin Z on carboxylated polyethylene glycol polymer cushion. Soft Matter 2011, 7, 7274–7280. [Google Scholar] [CrossRef]
- Hey, T.; Bajraktari, N.; Vogel, J.; Hélix Nielsen, C.; la Cour Jansen, J.; Jönsson, K. The effects of physicochemical wastewater treatment operations on forward osmosis. Environ. Technol. (UK) 2017, 38, 2130–2142. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Valladares Linares, R.; Bucs, S.; Fortunato, L.; Hélix-Nielsen, C.; Vrouwenvelder, J.S.; Ghaffour, N.; Leiknes, T.O.; Amy, G. Aquaporin based biomimetic membrane in forward osmosis: Chemical cleaning resistance and practical operation. Desalination 2017, 420, 208–215. [Google Scholar] [CrossRef]
- Song, H.; Xie, F.; Chen, W.; Liu, J. FO/MD hybrid system for real dairy wastewater recycling. Environ. Technol. (UK) 2018, 39, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.J.A.; Chirwa, E.M.N. The viability of forward osmosis in the concentration of biologically produced fumaric acid using l-alanine as a draw solution. Chem. Eng. Trans. 2018, 64, 259–264. [Google Scholar]
- Xue, W.; Sint, K.K.K.; Ratanatamskul, C.; Praserthdam, P.; Yamamoto, K. Binding TiO2 nanoparticles to forward osmosis membranes: Via MEMO-PMMA-Br monomer chains for enhanced filtration and antifouling performance. RSC Adv. 2018, 8, 19024–19033. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Xie, M.; Song, X.; Guo, W.; Ngo, H.H.; Zhou, J.L.; Nghiem, L.D. Biomimetic aquaporin membranes for osmotic membrane bioreactors: Membrane performance and contaminant removal. Bioresour. Technol. 2018, 249, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Petrinic, I.; Hélix-Nielsen, C.; Basu, S.; Balakrishnan, M. Concentrating molasses distillery wastewater using biomimetic forward osmosis (FO) membranes. Water Res. 2018, 130, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Soler-Cabezas, J.L.; Mendoza-Roca, J.A.; Vincent-Vela, M.C.; Luján-Facundo, M.J.; Pastor-Alcañiz, L. Simultaneous concentration of nutrients from anaerobically digested sludge centrate and pre-treatment of industrial effluents by forward osmosis. Sep. Purif. Technol. 2018, 193, 289–296. [Google Scholar] [CrossRef]
- Kalafatakis, S.; Braekevelt, S.; Lymperatou, A.; Zarebska, A.; Hélix-Nielsen, C.; Lange, L.; Skiadas, I.V.; Gavala, H.N. Application of forward osmosis technology in crude glycerol fermentation biorefinery-potential and challenges. Bioprocess Biosyst. Eng. 2018, 41, 1089–1101. [Google Scholar] [CrossRef]
- Munshi, F.M.; Church, J.; McLean, R.; Maier, N.; Sadmani, A.H.M.A.; Duranceau, S.J.; Lee, W.H. Dewatering algae using an aquaporin-based polyethersulfone forward osmosis membrane. Sep. Purif. Technol. 2018, 204, 154–161. [Google Scholar] [CrossRef]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar]
- Barone, L.M.; Mu, H.H.; Shih, C.J.; Kashlan, K.B.; Wasserman, B.P. Distinct biochemical and topological properties of the 31-and 27-kilodalton plasma membrane intrinsic protein subgroups from red beet. Plant Physiol. 1998, 118, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Cabañero, F.; Olmos, E.; Periago, P.M.; Maurel, C.; Carvajal, M. Two different effects of calcium on aquaporins in salinity-stressed pepper plants. Planta 2008, 228, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.Y.; Rahardianto, A.; Lee, R.W.; Cohen, Y. Morphometric characterization of calcium sulfate dihydrate (gypsum) scale on reverse osmosis membranes. J. Memb. Sci. 2005, 252, 253–263. [Google Scholar] [CrossRef]
- Lin, N.H.; Cohen, Y. QCM study of mineral surface crystallization on aromatic polyamide membrane surfaces. J. Memb. Sci. 2011, 379, 426–433. [Google Scholar] [CrossRef]
- Xie, M.; Gray, S.R. Gypsum scaling in forward osmosis: Role of membrane surface chemistry. J. Memb. Sci. 2016, 513, 250–259. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Tousley, M.E.; Elimelech, M. Influence of polyamide membrane surface chemistry on gypsum scaling behavior. J. Memb. Sci. 2017, 525, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Mi, B.I.; Elimelech, M. Gypsum scaling and cleaning in forward osmosis: Measurements and mechanisms. Environ. Sci. Technol. 2010, 44, 2022–2028. [Google Scholar] [CrossRef]
- Balkenov, A.; Anuarbek, A.; Satayeva, A.; Kim, J.; Inglezakis, V.; Arkhangelsky, E. Complex organic fouling and effect of silver nanoparticles on aquaporin forward osmosis membranes. J. Water Process Eng. 2020, 34, 101177. [Google Scholar] [CrossRef]
- Wang, K.; Xu, L.; Li, K.; Liu, L.; Zhang, Y.; Wang, J. Development of polyaniline conductive membrane for electrically enhanced membrane fouling mitigation. J. Memb. Sci. 2019, 570–571, 371–379. [Google Scholar] [CrossRef]
- Xia, L.; Andersen, M.F.; Hélix-Nielsen, C.; McCutcheon, J.R. Novel Commercial Aquaporin Flat-Sheet Membrane for Forward Osmosis. Ind. Eng. Chem. Res. 2017, 56, 11919–11925. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani, J.J.; Malamis, S.; Ochsenkühn-Petropoulou, M. Structural and chemical characterization of long-term reverse osmosis membrane fouling in a full scale desalination plant. Desalination 2012, 305, 44–53. [Google Scholar] [CrossRef]
- Kaufmann, R.S. Fick’s law. In Geochemistry. Encyclopedia of Earth Science; Marshall, C.P., Fairbridge, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; p. 245. ISBN 978-0-412-75500-2. [Google Scholar]
- Zou, S.; Gu, Y.; Xiao, D.; Tang, C.Y. The role of physical and chemical parameters on forward osmosis membrane fouling during algae separation. J. Memb. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Sheikholeslami, R. Mixed salts-Scaling limits and propensity. Desalination 2003, 154, 117–127. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, B.; Yue, Q.; Liu, S.; Shon, H.K. Effect of high salinity on the performance of forward osmosis: Water flux, membrane scaling and removal efficiency. Desalination 2016, 378, 67–73. [Google Scholar] [CrossRef]
- Majeed, T.; Phuntsho, S.; Sahebi, S.; Kim, J.E.; Yoon, J.K.; Kim, K.; Shon, H.K. Influence of the process parameters on hollow fiber-forward osmosis membrane performances. Desalin. Water Treat. 2015, 54, 817–828. [Google Scholar] [CrossRef]
- Jung, D.H.; Lee, J.; Kim, D.Y.; Lee, Y.G.; Park, M.; Lee, S.; Yang, D.R.; Kim, J.H. Simulation of forward osmosis membrane process: Effect of membrane orientation and flow direction of feed and draw solutions. Desalination 2011, 277, 83–91. [Google Scholar] [CrossRef]
- Chun, Y.; Qing, L.; Sun, G.; Bilad, M.R.; Fane, A.G.; Chong, T.H. Prototype aquaporin-based forward osmosis membrane: Filtration properties and fouling resistance. Desalination 2018, 445, 75–84. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Wang, Z.; Wang, S.C. Formation, charged characteristic and BSA adsorption behavior of carboxymethyl chitosan/PES composite MF membrane. J. Memb. Sci. 2003, 217, 151–158. [Google Scholar] [CrossRef]
- Gehr, R.; Zhai, Z.A.; Finch, J.A.; Rao, S.R. Reduction of soluble mineral concentrations in CaSO4 saturated water using a magnetic field. Water Res. 1995, 29, 933–940. [Google Scholar] [CrossRef]
- Hancer, M.; Celik, M.S.; Miller, J.D. The significance of interfacial water structure in soluble salt flotation systems. J. Colloid Interface Sci. 2001, 235, 150–161. [Google Scholar] [CrossRef]
- Genestar, C. Characterization of grounds used in canvas and sculpture. Mater. Lett. 2002, 54, 382–388. [Google Scholar] [CrossRef]
- Ray, J.R.; Wong, W.; Jun, Y.S. Antiscaling efficacy of CaCO3 and CaSO4 on polyethylene glycol (PEG)-modified reverse osmosis membranes in the presence of humic acid: Interplay of membrane surface properties and water chemistry. Phys. Chem. Chem. Phys. 2017, 19, 5647–5657. [Google Scholar] [CrossRef] [PubMed]


















| Feed Solution (g/L) | ||||
|---|---|---|---|---|
| SI | NaCl | CaCl2 | Na2SO4 | CaSO4 × 2H2O |
| Multi-component feed | ||||
| 1.5 | 1.188 | 4.153 | 3.039 | |
| 2 | 1.493 | 5.22 | 3.82 | |
| 2.45 | 1.754 | 6.32 | 4.487 | |
| 3 | 2.138 | 7.475 | 5.469 | |
| Single-component feed | ||||
| 1.5 | 2.756 | |||
| 2 | 3.47 | |||
| 2.45 | 4.078 | |||
| Concentrations (wt. %) | ||
|---|---|---|
| Element | Intact | Scaled |
| S | 87.47 | 29.58 |
| Cl | 4.27 | 6.56 |
| Ti | 3.57 | 0.43 |
| Ca | 2.97 | 63.59 |
| K | 0.46 | --- |
| Fe | 0.42 | 0.17 |
| Si | 0.15 | --- |
| Mg | 0.13 | 0.09 |
| Ni | 0.10 | 0.05 |
| Cu | 0.07 | --- |
| Zn | 0.03 | --- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omir, A.; Satayeva, A.; Chinakulova, A.; Kamal, A.; Kim, J.; Inglezakis, V.J.; Arkhangelsky, E. Behaviour of Aquaporin Forward Osmosis Flat Sheet Membranes during the Concentration of Calcium-Containing Liquids. Membranes 2020, 10, 108. https://doi.org/10.3390/membranes10050108
Omir A, Satayeva A, Chinakulova A, Kamal A, Kim J, Inglezakis VJ, Arkhangelsky E. Behaviour of Aquaporin Forward Osmosis Flat Sheet Membranes during the Concentration of Calcium-Containing Liquids. Membranes. 2020; 10(5):108. https://doi.org/10.3390/membranes10050108
Chicago/Turabian StyleOmir, Alibek, Aliya Satayeva, Aigerim Chinakulova, Arailym Kamal, Jong Kim, Vassilis J. Inglezakis, and Elizabeth Arkhangelsky. 2020. "Behaviour of Aquaporin Forward Osmosis Flat Sheet Membranes during the Concentration of Calcium-Containing Liquids" Membranes 10, no. 5: 108. https://doi.org/10.3390/membranes10050108