Membrane Separation Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: A Short Review
Abstract
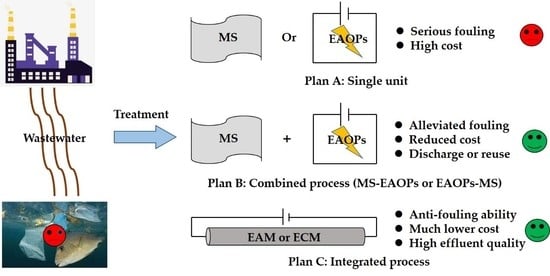
:1. Introduction
2. Coupling MS and EAOPs as a Combined Process
2.1. MS Combined with EAOPs
2.1.1. MS as Post-Treatment after EAOPs (EAOPs-MS)
2.1.2. MS as Pretreatment Followed by EAOPs (MS-EAOPs)
2.2. MBRs Combined with EO
3. Coupling MS and Anodic EAOPs as an Integrated Technology
3.1. Development of EAM Technology
3.2. Advantages and Mechanisms of REM
3.2.1. Enhancement of Mass Transfer and Electroactive Surface Area
3.2.2. Mechanism of Antifouling and Membrane Regeneration
- Repulsion between the foulant and membrane occurs in the presence of an electric field;
- The diffusion of reactive oxygen species (ROS) and active chlorine (AC) generated by the EO process to the vicinity of the membrane surface helps in membrane cleaning by reacting with foulants;
- ROS and AC produced in the membrane lumen can achieve in situ membrane cleaning;
- Foulants are degraded by EO into small molecules that mitigate membrane fouling.
3.3. Application of REMs
4. Coupling MS and Cathodic EAOPs as an Integrated Technology
4.1. Development of ECM Technology
4.2. Reaction Mechanism of ECM Technologies
4.2.1. CEFMs
4.2.2. GDCs
4.3. Application of ECMs
5. Conclusions and Prospects
- Development of suitable electrode and membrane materials to acquire better catalytic and physical properties (e.g., OEP, conductivity, corrosion resistance, and impedance);
- Comprehensive study of coupling mechanism of two technologies to clarify the interaction to explore the optimum operating conditions;
- Deep understanding of fluid dynamic in the coupling system during the operation, better to do modeling analysis by computer flow dynamic (CFD) study;
- Necessary engineering optimization of arrangement of facility to simplify the implementation.
Author Contributions
Funding
Conflicts of Interest
References
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Song, C.; Li, L.; Wang, H.; Pan, Y.; Wang, C.; Li, J.; Wang, T.; Feng, X. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chem. Eng.J. 2019, 376, 120909–120928. [Google Scholar] [CrossRef]
- Shafieian, A.; Khiadani, M.; Azhar, M.R. A solar membrane-based wastewater treatment system for high-quality water production. Energy 2020, 206, 118233–118247. [Google Scholar] [CrossRef]
- Skolotneva, E.; Trellu, C.; Cretin, M.; Mareev, S. A 2D convection-diffusion model of anodic oxidation of organic compounds mediated by hydroxyl radicals using porous reactive electrochemical membrane. Membranes 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Rivallin, M.; Cerneaux, S.; Coetsier, C.; Causserand, C.; Oturan, M.A.; Cretin, M. Integration of sub-stoichiometric titanium oxide reactive electrochemical membrane as anode in the electro-Fenton process. Chem. Eng. J. 2020, 400, 125936–125944. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. Int. Ed. Engl. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Pérez, S.; Rubiano, M.-E.; Ginebreda, A.; Postigo, C.; López-Serna, R.; Blanco, J.; Osorio, V.; de Alda, M.L.; Petrović, M.; Pastor, J.J.; et al. Wastewater reuse in the Llobregat: The experience at the Prat de Llobregat treatment plant. Hdb. Env. Chem. 2012, 21, 327–346. [Google Scholar]
- Filloux, E.; Gernjak, W.; Gallard, H.; Croue, J.P. Investigating the relative contribution of colloidal and soluble fractions of secondary effluent organic matter to the irreversible fouling of MF and UF hollow fibre membranes. Sep. Purif. Technol. 2016, 170, 109–115. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Ed. Engl. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, Y.; Han, W.; Li, J.; Sun, X.; Shen, J.; Wang, L. Advanced treatment of triazole fungicides discharged water in pilot scale by integrated system: Enhanced electrochemical oxidation, upflow biological aerated filter and electrodialysis. Chem. Eng. J. 2017, 315, 335–344. [Google Scholar] [CrossRef]
- Ling, Y.; Hu, J.; Qian, Z.; Zhu, L.; Chen, X. Continuous treatment of biologically treated textile effluent using a multi-cell electrochemical reactor. Chem. Eng. J. 2016, 286, 571–577. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Xekoukoulotakis, N.P.; Coz, A.; Kalogerakis, N.; Mantzavinos, D. Electrochemical treatment of textile dyes and dyehouse effluents. J. Hazard. Mater. 2006, 137, 998–1007. [Google Scholar] [CrossRef]
- Moreira, F.C.; Soler, J.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Incorporation of electrochemical advanced oxidation processes in a multistage treatment system for sanitary landfill leachate. Water Res. 2015, 81, 375–387. [Google Scholar] [CrossRef]
- He, Y.; Dong, Y.; Huang, W.; Tang, X.; Liu, H.; Lin, H.; Li, H. Investigation of boron-doped diamond on porous Ti for electrochemical oxidation of acetaminophen pharmaceutical drug. J. Electroanal. Chem. 2015, 759, 167–173. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, L.; Han, W.; Wang, L.; Sun, X.; Li, J. Treatment of high explosive production wastewater containing RDX by combined electrocatalytic reaction and anoxic–oxic biodegradation. Chem. Eng. J. 2011, 168, 1256–1262. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Song, C.; Tao, P.; Sun, M.; Shao, M.; Wang, T. Enhanced treatment ability of membrane technology by integrating an electric field for dye wastewater treatment: A review. J. AOAC Int. 2018, 101, 1341–1352. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Wang, H.; Song, X.; Wang, T.; He, B.; Liang, X.; Ngo, H.H. An electrocatalytic membrane reactor with self-cleaning function for industrial wastewater treatment. Angew. Chem. Int. Ed. Engl. 2011, 123, 2196–2198. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Z.; Zhu, X.; Fan, Y.; Wang, W. Advanced treatment of textile wastewater for reuse using electrochemical oxidation and membrane filtration. Water SA 2005, 31, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Masid, S.; Waghmare, S.; Gedam, N.; Misra, R.; Dhodapkar, R.; Nandy, T.; Rao, N.N. Impact of electrooxidation on combined physicochemical and membrane treatment processes: Treatment of high strength chemical industry wastewater. Desalination 2010, 259, 192–196. [Google Scholar] [CrossRef]
- Diogo, J.C.; Morão, A.; Lopes, A. Persistent aromatic pollutants removal using a combined process of electrochemical treatment and reverse osmosis/nanofiltration. Environ. Prog. Sustain. 2011, 30, 399–408. [Google Scholar] [CrossRef]
- Xu, L.; Du, L.-S.; Wang, C.; Xu, W. Nanofiltration coupled with electrolytic oxidation in treating simulated dye wastewater. J. Membr. Sci. 2012, 409–410, 329–334. [Google Scholar] [CrossRef]
- Juang, Y.; Nurhayati, E.; Huang, C.; Pan, J.R.; Huang, S. A hybrid electrochemical advanced oxidation/microfiltration system using BDD/Ti anode for acid yellow 36 dye wastewater treatment. Sep. Purif. Technol. 2013, 120, 289–295. [Google Scholar] [CrossRef]
- Madsen, H.T.; Søgaard, E.G.; Muff, J. Reduction in energy consumption of electrochemical pesticide degradation through combination with membrane filtration. Chem. Eng. J. 2015, 276, 358–364. [Google Scholar] [CrossRef]
- Mameda, N.; Park, H.J.; Choo, K.H. Membrane electro-oxidizer: A new hybrid membrane system with electrochemical oxidation for enhanced organics and fouling control. Water Res. 2017, 126, 40–49. [Google Scholar] [CrossRef]
- Du, X.; Yang, W.; Zhao, J.; Zhang, W.; Cheng, X.; Liu, J.; Wang, Z.; Li, G.; Liang, H. Peroxymonosulfate-assisted electrolytic oxidation/ coagulation combined with ceramic ultrafiltration for surface water treatment: Membrane fouling and sulfamethazine degradation. J. Clean. Prod. 2019, 235, 779–788. [Google Scholar] [CrossRef]
- Acosta-Santoyo, G.; Llanos, J.; Raschitor, A.; Bustos, E.; Cañizares, P.; Rodrigo, M.A. Performance of ultrafiltration as a pre-concentration stage for the treatment of oxyfluorfen by electrochemical BDD oxidation. Sep. Purif. Technol. 2020, 237, 116366–116371. [Google Scholar] [CrossRef]
- Mostafazadeh, A.K.; Benguit, A.T.; Carabin, A.; Drogui, P.; Brien, E. Development of combined membrane filtration, electrochemical technologies, and adsorption processes for treatment and reuse of laundry wastewater and removal of nonylphenol ethoxylates as surfactants. J. Water Process. Eng. 2019, 28, 277–292. [Google Scholar] [CrossRef]
- Perez, G.; Fernandez-Alba, A.R.; Urtiaga, A.M.; Ortiz, I. Electro-oxidation of reverse osmosis concentrates generated in tertiary water treatment. Water Res. 2010, 44, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Bagastyo, A.Y.; Batstone, D.J.; Kristiana, I.; Gernjak, W.; Joll, C.; Radjenovic, J. Electrochemical oxidation of reverse osmosis concentrate on boron-doped diamond anodes at circumneutral and acidic pH. Water Res. 2012, 46, 6104–6112. [Google Scholar] [CrossRef] [PubMed]
- Hege, K.V.; Verhaege, M.; Verstraete, W. Indirect electrochemical oxidation of reverse osmosis membrane concentrates at boron-doped diamond electrodes. Electrochem. Commun. 2002, 4, 296–300. [Google Scholar]
- Van Hege, K.; Verhaege, M.; Verstraete, W. Electro-oxidative abatement of low-salinity reverse osmosis membrane concentrates. Water Res. 2004, 38, 1550–1558. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Schrader, G.; Farrell, J. Electrochemical destruction of N-Nitrosodimethylamine in reverse osmosis concentrates using boron-doped diamond film electrodes. Environ. Sci. Technol. 2010, 44, 4264–4269. [Google Scholar] [CrossRef]
- Soriano, Á.; Gorri, D.; Urtiaga, A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 2017, 112, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Kateb, M.E.; Trellu, C.; Darwich, A.; Rivallin, M.; Bechelany, M.; Nagarajan, S.; Lacour, S.; Bellakhal, N.; Lesage, G.; Héran, M.; et al. Electrochemical advanced oxidation processes using novel electrode materials for mineralization and biodegradability enhancement of nanofiltration concentrate of landfill leachates. Water Res. 2019, 162, 446–455. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Chung, C.M.; Tobino, T.; Cho, K.; Yamamoto, K. Alleviation of membrane fouling in a submerged membrane bioreactor with electrochemical oxidation mediated by in-situ free chlorine generation. Water Res. 2016, 96, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Ncibi, M.C.; Shestakova, M.; Sillanpää, M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl. Catal. B Environ. 2018, 221, 329–338. [Google Scholar] [CrossRef]
- Zhang, S.; Van Houten, R.; Eikelboom, D.H.; Doddema, H.; Jiang, Z.; Fan, Y.; Wang, J. Sewage treatment by a low energy membrane bioreactor. Bioresour. Technol. 2003, 90, 185–192. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Jia, J.P. Improvement of electrochemical wastewater treatment through mass transfer in a seepage carbon nanotube electrode reactor. Environ. Sci. Technol. 2009, 43, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.M.; Chaplin, B.P. Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment. Environ. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wang, H.; Cheng, B.; He, B.; Yan, F.; Yang, Y.; Guo, W.; Ngo, H.H. Electrocatalytic oxidation of n-propanol to produce propionic acid using an electrocatalytic membrane reactor. Chem. Commun. (Camb) 2013, 49, 4501–4503. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Environ. Sci. Technol. 2014, 48, 5857–5867. [Google Scholar] [CrossRef]
- Wang, H.; Guan, Q.; Li, J.; Wang, T. Phenolic wastewater treatment by an electrocatalytic membrane reactor. Catal. Today 2014, 236, 121–126. [Google Scholar] [CrossRef]
- Guo, L.; Jing, Y.; Chaplin, B.P. Development and characterization of ultrafiltration TiO2 Magnéli Phase reactive electrochemical membranes. Environ. Sci. Technol. 2016, 50, 1428–1436. [Google Scholar] [CrossRef]
- Guo, L.; Ding, K.; Rockne, K.; Duran, M.; Chaplin, B.P. Bacteria inactivation at a sub-stoichiometric titanium dioxide reactive electrochemical membrane. J. Hazard. Mater. 2016, 319, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Gayen, P.; Spataro, J.; Avasarala, S.; Ali, A.-M.; Cerrato, J.M.; Chaplin, B.P. Electrocatalytic reduction of nitrate using Magnéli Phase TiO2 reactive electrochemical membranes doped with Pd-based catalysts. Environ. Sci. Technol. 2018, 52, 9370–9379. [Google Scholar] [CrossRef] [PubMed]
- Gayen, P.; Chen, C.; Abiade, J.T.; Chaplin, B.P. Electrochemical oxidation of atrazine and clothianidin on Bi-doped SnO2–TinO2n–1 electrocatalytic reactive electrochemical membranes. Environ. Sci. Technol. 2018, 52, 12675–12684. [Google Scholar] [CrossRef] [PubMed]
- Almassi, S.; Li, Z.; Xu, W.; Pu, C.; Zeng, T.; Chaplin, B.P. Simultaneous adsorption and electrochemical reduction of N‑Nitrosodimethylamine using Carbon-Ti4O7 composite reactive electrochemical membranes. Environ. Sci. Technol. 2019, 53, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.H.; Haflich, H.; Shah, A.D.; Chaplin, B.P. Energy-Efficient electrochemical oxidation of perfluoroalkyl substances using a Ti4O7 reactive electrochemical membrane anode. Environ. Sci. Technol. Lett. 2019, 6, 504–510. [Google Scholar] [CrossRef]
- Almassi, S.; Samonte, P.R.V.; Li, Z.; Xu, W.; Chaplin, B.P. Mechanistic investigation of haloacetic acid reduction using Carbon-Ti4O7 composite reactive electrochemical membranes. Environ. Sci. Technol. 2019, 54, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Misal, S.N.; Lin, M.H.; Mehraeen, S.; Chaplin, B.P. Modeling electrochemical oxidation and reduction of sulfamethoxazole using electrocatalytic reactive electrochemical membranes. J. Hazard. Mater. 2020, 384, 121420–121431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, K.; Han, W.; Sun, X.; Li, J.; Shen, J.; Wang, L. Improved electrochemical oxidation of tricyclazole from aqueous solution by enhancing mass transfer in a tubular porous electrode electrocatalytic reactor. Electrochim. Acta 2016, 189, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Han, W.; Sun, X.; Li, J.; Shen, J.; Wang, L. Electrochemical treatment of anticancer drugs wastewater containing 5-Fluoro-2-Methoxypyrimidine using a tubular porous electrode electrocatalytic reactor. Electrochim. Acta 2016, 220, 211–221. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.; Zhou, X.; Li, J.; Sun, X.; Shen, J.; Wang, L.; Han, W. Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, K.; Xu, A.; Han, W.; Sun, X.; Li, J.; Shen, J.; Wang, L. Pesticide tailwater deeply treated by tubular porous electrode reactor (TPER): Purpose for discharging and cost saving. Chemosphere 2017, 185, 86–93. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Zhou, X.; Han, W.; Li, J.; Sun, X.; Shen, J.; Wang, L. Improved degradation of the aqueous flutriafol using a nanostructure macroporous PbO2 as reactive electrochemical membrane. Electrochim. Acta 2017, 253, 357–367. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Xu, A.; Wei, K.; Han, W.; Li, J.; Sun, X.; Shen, J.; Liu, X.; Wang, L. A multi-walled carbon nanotube electrode based on porous Graphite-RuO2 in electrochemical filter for pyrrole degradation. Chem. Eng. J. 2017, 330, 956–964. [Google Scholar] [CrossRef]
- Liu, S.; Cui, T.; Xu, A.; Han, W.; Li, J.; Sun, X.; Shen, J.; Wang, L. Electrochemical treatment of flutriafol wastewater using a novel 3D macroporous PbO2 filter: Operating parameters, mechanism and toxicity assessment. J. Hazard. Mater. 2018, 358, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, G.; Shi, M.; Li, J.; Li, J.; Guo, C.; Lee, J.K.; Zheng, J. Using TiO2 mesoflower interlayer in tubular porous titanium membranes for enhanced electrocatalytic filtration. Electrochim. Acta 2016, 218, 318–324. [Google Scholar] [CrossRef]
- Sun, M.; Feng, G.; Zhang, M.; Song, C.; Tao, P.; Wang, T.; Shao, M. Enhanced removal ability of phenol from aqueous solution using coal-based carbon membrane coupled with electrochemical oxidation process. Colloid Surface A 2018, 540, 186–193. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Dai, R.; Wu, Z.; Liu, M.; Wang, Z. Development of a moving-bed electrochemical membrane bioreactor to enhance removal of low-concentration antibiotic from wastewater. Bioresour. Technol. 2019, 293, 122022–122030. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.C.W.; Dionysiou, D.D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2020, 383, 123149–123157. [Google Scholar] [CrossRef]
- Sergienko, N.; Radjenovic, J. Manganese oxide-based porous electrodes for rapid and selective (electro)catalytic removal and recovery of sulfide from wastewater. Appl. Catal. B Environ. 2020, 267, 118608–118615. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Ma, J.; Xu, S.; Wu, Z. Development of an electrochemical ceramic membrane filtration system for efficient contaminant removal from waters. Environ. Sci. Technol. 2018, 52, 4117–4126. [Google Scholar] [CrossRef]
- Hua, L.; Cao, H.; Ma, Q.; Shi, X.; Zhang, X.; Zhang, W. Microalgae filtration using an electrochemically reactive ceramic membrane: Filtration performances, fouling kinetics, and foulant layer characteristics. Environ. Sci. Technol. 2020, 54, 2012–2021. [Google Scholar] [CrossRef]
- Jing, Y.; Chaplin, B.P. Electrochemical impedance spectroscopy study of membrane fouling characterization at a conductive sub-stoichiometric TiO2 reactive electrochemical membrane: Transmission line model development. J. Membr. Sci. 2016, 511, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Guo, L.; Chaplin, B.P. Electrochemical impedance spectroscopy study of membrane fouling and electrochemical regeneration at a sub-stoichiometric TiO2 reactive electrochemical membrane. J. Membr. Sci. 2016, 510, 510–523. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ji, Q.; Liu, H.; Qu, J. Pore structure-dependent mass transport in flow-through electrodes for water remediation. Environ. Sci. Technol. 2018, 52, 7477–7485. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Rodrigo, M.A.; Sires, I.; Scialdone, O. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: A critical review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Griffiths, M.; de León, C.P.; Walsh, F.C. Mass transport in the rectangular channel of a filter-press electrolyzer (the FM01-LC reactor). AlChE J. 2005, 51, 682–687. [Google Scholar] [CrossRef]
- Ralph, T.R.; Hitchman, M.L.; Millington, J.P.; Walsh, F.C. Mass transport in an electrochemical laboratory filterpress reactor and its enhancement by turbulence promoters. Electrochim. Acta 1996, 41, 591–603. [Google Scholar] [CrossRef]
- Shukla, P.; Singh, K.K.; Tewari, P.K.; Gupta, P.K. Numerical simulation of flow electrolysers: Effect of obstacles. Electrochim. Acta 2012, 79, 57–66. [Google Scholar] [CrossRef]
- Wei, X.; Wang, H.; Yin, Z.; Qaseem, S.; Li, J. Tubular electrocatalytic membrane reactor for alcohol oxidation: CFD simulation and experiment. Chin. J. Chem. Eng. 2017, 25, 18–25. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.N.; Bisang, J.M. The effect of a perpendicular and cumulative inlet flow on the mass-transfer distribution in parallel-plate electrochemical reactors. Electrochim. Acta 2014, 137, 758–766. [Google Scholar] [CrossRef]
- Montilla, F.; Morallon, E.; Battisti, A.D.; Vazquez, J.L. Preparation and characterization of antimony-doped tin dioxide electrodes. Part 1. Electrochemical characterization. J. Phys. Chem. B 2004, 108, 213–217. [Google Scholar] [CrossRef]
- Zhao, W.; Xing, J.; Chen, D.; Bai, Z.; Xia, Y. Study on the performance of an improved Ti/SnO2–Sb2O3/PbO2 based on porous titanium substrate compared with planar titanium substrate. RSC Adv. 2015, 5, 26530–26539. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, L.; Xue, H.M.; Han, W.Q.; Wang, L.J.; Sun, X.Y.; Li, J.S. Preparation and characterization of TiO2-NTs/SnO2-Sb electrodes by electrode position. J. Electroanal. Chem. 2010, 648, 119–127. [Google Scholar] [CrossRef]
- Liu, H.; Vecitis, C.D. Reactive transport mechanism for organic oxidation during electrochemical filtration: Mass-transfer, physical adsorption, and electron-transfer. J. Phys. Chem. C 2011, 116, 374–383. [Google Scholar] [CrossRef]
- Bin, D.; Wang, H.; Li, J.; Wang, H.; Yin, Z.; Kang, J.; He, B.; Li, Z. Controllable oxidation of glucose to gluconic acid and glucaric acid using an electrocatalytic reactor. Electrochim. Acta 2014, 130, 170–178. [Google Scholar] [CrossRef]
- Wang, H.; Wei, X.; Zhang, Y.; Ma, R.; Yin, Z.; Li, J. Electrochemical analysis and convection-enhanced mass transfer synergistic effect of MnOx /Ti membrane electrode for alcohol oxidation. Chin. J. Chem. Eng. 2019, 27, 150–156. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, J.; He, B.; Wang, T.; Liao, S. Novel functionalized nano-TiO2 loading electrocatalytic membrane for oily wastewater treatment. Environ. Sci. Technol. 2012, 46, 6815–6821. [Google Scholar] [CrossRef]
- Azhar, M.R.; Arafat, Y.; Zhong, Y.; Khiadani, M.; Tade, M.O.; Wang, S.; Shao, Z. An adsorption−catalysis pathway toward sustainable application of mesoporous carbon nanospheres for efficient environmental remediation. ACS EST Water 2020, in press. [Google Scholar] [CrossRef]
- Fu, W.; Wang, X.; Zheng, J.; Liu, M.; Wang, Z. Antifouling performance and mechanisms in an electrochemical ceramic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 570–571, 355–361. [Google Scholar] [CrossRef]
- Bakr, A.R.; Rahaman, M.S. Removal of bisphenol A by electrochemical carbon-nanotube filter: Influential factors and degradation pathway. Chemosphere 2017, 185, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Guo, L.; Thakkar, M.; Wei, D.; Agbakpe, M.; Kuang, L.; Magpile, M.; Chaplin, B.P.; Tao, Y.; Shuai, D.; et al. Effects of anodic oxidation of a substoichiometric titanium dioxide reactive electrochemical membrane on algal cell destabilization and lipid extraction. Bioresour. Technol. 2016, 203, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Lin, H.; Habteselassie, M.; Huang, Q. Electrochemical inactivation of bacteria with a titanium sub-oxide reactive membrane. Water Res. 2018, 145, 172–180. [Google Scholar] [CrossRef]
- Huang, E.; White, T.; Wang, B.; Shi, H.; Liu, J. Disinfection of escherichia coli by a reactive electrochemical membrane system involving activated carbon fiber cloth (ACFC). Water 2019, 11, 430. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.; Zheng, J.; Ma, J.; Wang, X.; Wu, Z.; Wang, Z. Simultaneous solid-liquid separation and wastewater disinfection using an electrochemical dynamic membrane filtration system. Environ. Res. 2020, 180, 108861–108867. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.; Lin, H.; Xie, R.; Wang, K.; Lv, S.; Liao, J.; Liu, X.; Chen, J.; Yang, Z. Developing a low-pressure and super stable electrochemical tubular reactive filter: Outstanding efficiency for wastewater purification. Electrochim. Acta 2020, 335, 135634–135642. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Chen, J.; Niu, J. Porous Ti/SnO2-Sb anode as reactive electrochemical membrane for removing trace antiretroviral drug stavudine from wastewater. Environ. Int. 2019, 133, 105157–105164. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Q.; Zhang, J.; Wang, Z.; Wu, Z. Degradation of sulfadiazine in drinking water by a cathodic electrochemical membrane filtration process. Electrochim. Acta 2018, 277, 77–87. [Google Scholar] [CrossRef]
- Brillas, E.; Sire´s, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Ayadi, S.; Jedidi, I.; Rivallin, M.; Gillot, F.; Lacour, S.; Cerneaux, S.; Cretin, M.; Amar, R.B. Elaboration and characterization of new conductive porous graphite membranes for electrochemical advanced oxidation processes. J. Membr. Sci. 2013, 446, 42–49. [Google Scholar] [CrossRef]
- Gozzi, F.; Sirés, I.; de Oliveira, S.C.; Machulek, A.; Brillas, E. Influence of chelation on the Fenton-based electrochemical degradation of herbicide tebuthiuron. Chemosphere 2018, 199, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trellu, C.; Oturan, N.; Keita, F.K.; Fourdrin, C.; Pechaud, Y.; Oturan, M.A. Regeneration of activated carbon fiber by the electro-Fenton process. Environ. Sci. Technol. 2018, 52, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, Y.; Xiong, S.; Lin, P.; Hu, L.; Chen, S.; Wang, H.; Wang, L. A flexible and efficient electro-Fenton cathode film with aeration function based on polyphenylene sulfide ultra-fine fiber. React. Funct. Polym. 2019, 139, 42–49. [Google Scholar] [CrossRef]
- Brillas, E. A Review on the degradation of organic pollutants in waters by UV photoelectro‑Fenton and solar photoelectro‑Fenton. J. Braz. Chem. Soc. 2014, 25, 393–417. [Google Scholar] [CrossRef]
- Li, K.; Xu, L.; Zhang, Y.; Cao, A.; Wang, Y.; Huang, H.; Wang, J. A novel electro-catalytic membrane contactor for improving the efficiency of ozone on wastewater treatment. Appl. Catal. B Environ. 2019, 249, 316–321. [Google Scholar] [CrossRef]
- Rashid, M.H.O.; Pham, S.Q.T.; Sweetman, L.J.; Alcock, L.J.; Wise, A.; Nghiem, L.D.; Triani, G.; Panhuis, M.I.H.; Ralph, S.F. Synthesis, properties, water and solute permeability of MWNT buckypapers. J. Membr. Sci. 2014, 456, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xu, S.; Wu, Z.; Wang, Z. Removal of p-chloroaniline from polluted waters using a cathodic electrochemical ceramic membrane reactor. Sep. Purif. Technol. 2019, 211, 753–763. [Google Scholar] [CrossRef]
- Huong Le, T.X.; Dumée, L.F.; Lacour, S.; Rivallin, M.; Yi, Z.; Kong, L.; Bechelany, M.; Cretin, M. Hybrid graphene-decorated metal hollow fibre membrane reactors for efficient electro-Fenton–Filtration co-processes. J. Membr. Sci. 2019, 587, 117182–117189. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. Mitigating membrane fouling based on in situ ·OH Generation in a novel electro-Fenton membrane bioreactor. Environ. Sci. Technol. 2020, 54, 7669–7676. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Xu, L.; Liu, L.; Wang, Z.; Hou, D.; Wang, Y.; Wang, J. Mass transfer and interfacial reaction mechanisms in a novel electro-catalytic membrane contactor for wastewater treatment by O3. Appl. Catal. B Environ. 2020, 264, 118512–118519. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, M.; Li, Y.; Su, P.; Cai, J.; Pan, Y. Improving the yield of hydrogen peroxide on gas diffusion electrode modified with tert-butyl-anthraquinone on different carbon support. Electrochim. Acta 2019, 320, 134552–134564. [Google Scholar] [CrossRef]
- Ye, Z.; Guelfi, D.R.V.; Álvarez, G.; Alcaide, F.; Brillas, E. Enhanced electrocatalytic production of H2O2 at Co-based air-diffusion cathodes for the photoelectro-Fenton treatment of bronopol. Appl. Catal. B Environ. 2019, 247, 191–199. [Google Scholar] [CrossRef]
- Cui, L.; Huang, H.; Ding, P.; Zhu, S.; Jing, W.; Gu, X. Cogeneration of H2O2 and ·OH via a novel Fe3O4/MWCNTs composite cathode in a dual-compartment electro-Fenton membrane reactor. Sep. Purif. Technol. 2020, 237, 116380–116390. [Google Scholar] [CrossRef]
- Tang, Q.; Li, B.; Ma, W.; Gao, H.; Zhou, H.; Yang, C.; Gao, Y.; Wang, D. Fabrication of a double-layer membrane cathode based on modified carbon nanotubes for the sequential electro-Fenton oxidation of p-nitrophenol. Environ. Sci. Pollut. Res. 2020, 27, 18773–18783. [Google Scholar] [CrossRef]
- Liang, P.; Rivallin, M.; Cerneaux, S.; Lacour, S.; Petit, E.; Cretin, M. Coupling cathodic electro-Fenton reaction to membrane filtration for AO7 dye degradation: A successful feasibility study. J. Membr. Sci. 2016, 510, 182–190. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Q.; Hao, Z.; Vecitis, C.D. Carbon nanotube membrane stack for flow-through sequential regenerative electro-Fenton. Environ. Sci. Technol. 2015, 49, 2375–2383. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R.; Brillas, E.; Sirés, I. Electrochemical advanced oxidation of carbofuran in aqueous sulfate and/or chloride media using a flow cell with a RuO2-based anode and an air-diffusion cathode at pre-pilot scale. Chem. Eng. J. 2018, 335, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef]
- Belal, B.; Yuan, S.; Li, Z.; Wang, H.; Zuo, J.; Komarneni, S. Electro-peroxone treatment of Orange II dye wastewater. Water Res. 2013, 47, 6234–6243. [Google Scholar]
- Zhang, Y.; Zuo, S.; Zhang, Y.; Li, M.; Cai, J.; Zhou, M. Disinfection of simulated ballast water by a flow-through electro-peroxone process. Chem. Eng. J. 2018, 348, 485–493. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhen, L.; Zhang, H.; Zhang, Y.; Wang, C. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. J. Hazard. Mater. 2012, 229–230, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, S.; Qiu, C.; Wang, Y.; Pan, X.; Wang, J.; Wang, C.; Zuo, J. Effective degradation of refractory organic pollutants in landfill leachate by electro-peroxone treatment. Electrochim. Acta 2013, 102, 174–182. [Google Scholar] [CrossRef]
- Yao, W.; Fu, J.; Yang, H.; Yu, G.; Wang, Y. The beneficial effect of cathodic hydrogen peroxide generation on mitigating chlorinated by-product formation during water treatment. Water Res. 2019, 157, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Brillas, E.; Centellas, F.; Rodriguez, R.M.; Cabot, P.L.; Garrido, J.A.; Sires, I. Treatment of olive oil mill wastewater by single electrocoagulation with different electrodes and sequential electrocoagulation/electrochemical Fenton-based processes. J. Hazard. Mater. 2018, 347, 58–66. [Google Scholar] [CrossRef]
- Steter, J.R.; Brillas, E.; Sirés, I. Solar photoelectro-Fenton treatment of a mixture of parabens spiked into secondary treated wastewater effluent at low input current. Appl. Catal. B Environ. 2018, 224, 410–418. [Google Scholar] [CrossRef]
- Thiam, A.; Sires, I.; Salazar, R.; Brillas, E. On the performance of electrocatalytic anodes for photoelectro-Fenton treatment of synthetic solutions and real water spiked with the herbicide chloramben. J. Environ. Manag. 2018, 224, 340–349. [Google Scholar] [CrossRef]
- Thiam, A.; Sires, I.; Brillas, E. Treatment of a mixture of food color additives (E122, E124 and E129) in different water matrices by UVA and solar photoelectro-Fenton. Water Res. 2015, 81, 178–187. [Google Scholar] [CrossRef]
- Ridruejo, C.; Centellas, F.; Cabot, P.L.; Sires, I.; Brillas, E. Electrochemical Fenton-based treatment of tetracaine in synthetic and urban wastewater using active and non-active anodes. Water Res. 2018, 128, 71–81. [Google Scholar] [CrossRef] [Green Version]





| Type of REMs | Pore Size | Blank Control | Mass Transfer Enhancement | Electro-Active Area Increasement | Reference |
|---|---|---|---|---|---|
| A seepage carbon nanotube electrode | Without insulated porous membrane | 1.6-fold | [44] | ||
| Electrochemical carbon nanotube (CNT) filter | 40–50 μm | Same electrode in batch mode | 6-fold | [86] | |
| Substoichiometric titanium dioxide (Ti4O7) REM | 1–6 μm | Same electrode without permeate | 10-fold | 619-fold | [45] |
| MnO2/Ti REM | ≈10 μm | Same electrode without permeate | ≈4-fold 1 | [87] | |
| RuO2/Ti REM | 0.98 μm | Same electrode in batch mode | 2-fold | 2-fold | [57] |
| A multi-walled carbon nanotube/graphite-RuO2 REM | 22 µm | Same electrode without permeate | 4.6-fold | [62] | |
| MnOx/Ti REM | Same electrode without permeate | ≈3.8-fold | [88] |
| Type of REM | Treating Subject Properties | Operation Conditions | Performance | Current Efficiency (CE)/Energy Consumption (EC) | Reference |
|---|---|---|---|---|---|
| A seepage carbon nanotube electrode | Simulated dye wastewater containing 25–200 mg/L Reactive Brilliant Red X-3B | U = 5–15 V Celectrolyte = 0–3 gL−1 Na2SO4 pH = 2–10V = 80.2 mLmin−1 | Total color and COD were removed by 94.4% and 57.6%, respectively in 90 min, much higher than that of 32.8–37.4% and 28.0–32.7% removal by conventional electrochemical processes | CE = 33.1% at time of 45 min while others were 7.5%, and 5.3% EC = 101.34 kWh kg−1COD | [44] |
| TiO2/Carbon REM | Oily water with concentration of 200 mg/L | U = 2.0 V I = 10.0 mA Celectrolyte = 15 g L−1 Na2SO4 Q = 100 Lm−2h−1bar−1 | Oil and COD removal were up to 86.2% and 94.4%, higher than original carbon membrane and TiO2/carbon membrane | EC = 0.166 kWh per ton of water | [21] |
| Simulated phenolic wastewater with concentration of 10 mM | Celectrolyte = 15 g L−1 Na2SO4 J = 0.3 mA cm−2 pH = 6 Rt = 0–5.2 min | A high phenol removal rate and complete mineralization fraction of 99.96 and 72.4% were achieved | [48] | ||
| MnO2/Ti REM | Producing propionic acid by oxidation of n-propanol (160 ± 5 mmol L−1) | U = 2.8 V Celectrolyte = 15 g L−1 Na2SO4 T = 25 and 50 °C Rt = 0–22.55 min | n-propanol conversion and the selectivity to propionic acid were improved to 60.77% and 56.82% when Rt increased from 0 min to 22.55 min, and meanwhile their value were 98.44% and 79.33% when T raising from 25 to 50 °C | [46] | |
| Substoichiometric TiO2 REM | Simulated industrial wastewater containing 1 mM p-methoxyphenol (p-MP) | Celectrolyte = 10 mM Na2SO4 J = 0–1.0 mA cm−2 V = 600 mL min−1 T = 21 ± 2 °C | Best p-MP and COD removal rate were 99.9 ± 0.17% and 30.1 ± 3.1% | CE > 73.3 % and best CE was 99.0 % at 0.5 mA cm−2 | [45] |
| Ultrafiltration TiO2 Magneéli Phase REM | Simulated wastewater containing 1 mM Oxalic Acid; Another Simulated wastewater containing 9 mM ClO4− and 10 mM NO3− | For oxidation of OA: U = 2.94 V Q = 390 LMH T = 21 °C Celectrolyte = 10 mM Na2SO4 For separation of Oxyanions: U = 0–10 V QJ = 58 and 1291 LMH | The optimal removal rate for oxalic acid was 401.5 ± 18.1 mmol h−1m−2 at 793 LMH; The removal rate of oxyanion was 67% at 58 LMH | EC for separation of oxyanions was 0.22 kWh m−3 | [49] |
| Multi-walled carbon nanotubes (MWCNTs)–Ti4O7 Composite REM | Synthetic solutions containing 10 μM or 150 μM N-nitrosodimethylamine (NDMA) | U = −1.1 V/SHE cathodic potential Q = 100 or 200 LMH Celectrolyte = 10 mM NaH2BO3 pH = 8.0 ± 0.1 | For 10 μM NDMA, the removal rate was below the HPLC method detection limit (0.1 μM), and GC/MS gives a value of approximate 4-log removal (99%); For 10 mM NDMA, the removal rate was 82.5 ± 1% | EC values were 0.12 ± 0.03 kWh m−3 and 0.58 ± 0.02 kWh m−3, respectively for 10 and 150 μM NDMA | [53] |
| RuO2/Ti REM | Simulated wastewater containing 20–100 mg/L Tricyclazole | J = 0–20 mA cm−2 Celectrolyte = 5 g L−1 Na2SO4 pH = 7 T = 20 °C V = 8 mL s−1 | The removal rate of Tricyclazole was approximate 100% at each Cinitial, all higher than conventional plate electrode | Best CE was 61.07% at 3 mA cm−2 | [57] |
| Actual anticancer drugs wastewater containing 61.2 mg L−1 5-Fluoro-2-Methoxypyrimidine | V = 0.08–0.31 mLmin−1 pH = 2–9 Celectrolyte = 0–7.5 g L−1 Na2SO4 J = 3–5 mA cm−2 | COD and 5-Fluoro-2-Methoxypyrimidine of the wastewater were removed by 84.1% and 100% at optimal condition, while BOD5/COD value and EC50,48h value were increased from 0.14 and 16.4% to 0.53 and 51.2%, respectively | EC = 1.5 kWh kg−1 COD | [58] | |
| Actual triazole fungicides discharged water in pilot scale (capacity of 10 m3 d−1) containing 150–200 mg L−1 Tricyclazole, 50–75 mg L−1 1H-1,2,4-Triazole and 25–55 mg L−1 Propiconazole | J = 1.5–5.5 mA cm−2 pH = 3–9 V = 3 m3 h−1 | Tricyclazole, 1H-1,2,4-Triazole and Propiconazole were removed by 94.19%, 90.11% and 100%, the COD of discharged water was removed by 53.06%, while the BOD5/COD ratio raised from 0.028 to 0.46 | Operation cost was 0.85 $ (m−3 d−1) | [14] | |
| Boron-doped multi-walled carbon nanotubes REM | Simulated wastewater containing 1 mg L−1 bisphenol A | U = 0 and 3 V Celectrolyte = 10 mM Na2SO4 pH = 3–9 V = 2 mL min−1 | Nearly complete removal of 1 mg L−1bisphenol A at 2 and 3 V of applied DC potentials was achieved | CE was ranged from 120 to 140 %, while EC was ranged from 15 to 50 KWh Kg−1 under different operation conditions. | [92] |
| Graphite–REM | Simulated drinking water containing 0.4–40 mM sulfadiazine (SDZ) and natural waters containing 40 mM SDZ | U = 0.5–3.0 V Q = 25, 50, and 75 LMH Celectrolyte = 50 mM Na2SO4 pH = 7 | For simulated drinking water, SDZ can be removed by approximate 100% at 3V. However, degradation rate of flow mode slower than batch and circulation mode, but it degraded more SDZ on the base of mass balance calculations; For natural waters containing SDZ, 79% SDZ was removed | EC value ranging from 0.007 to 0.39kWh m−3 for different voltages (0.5–3.0 V), and 0.14 to 0.37 kWh m−3 for different fluxes (25–75 LWH) | [99] |
| Bi-doped SnO2−TinO2n−1 REM | Simulated wastewater containing 1mM Terephthalic acid (TA), 10 μM Atrazine (ATZ) and 10 μM Clothianidin (CDN) | U = 2.1–3.5 V/SHE V = 0.5 mL min−1 T = 21 ± 2 °C Celectrolyte = 10 mM KH2PO4 pH = 4.5 | TA and COD conversion were achieved > 99.9% and > 97% at 3.5 V; ATZ conversion and %N mineralization were achieved > 99.9% and 91.3% at 3.5 V; CDN conversion and %N mineralization were achieved > 99.9 and 96.5% at 3.5 V | The minimal EC values per log removal of < 0.53 kWh m−3 for TA, < 0.42 kWh m−3 for ATZ, and 0.83 kWh m−3 for CDN | [52] |
| TiO2@SnO2−Sb/Ceramic REM | Simulated wastewater containing 10 μM p-chloroaniline(PCA) | U = 1–5 V T = 25 ± 1 °C pH = 7.0 Celectrolyte = 50 mM Na2SO4 Q = 11.6−138.9 Lm−2 h−1 | PCA was removed by 97.9% at voltage of 5V with flux of 17.4 L m−2 h−1 in flow-through mode, 1.9 times than that of flow-by mode. In addition, either the removal rate or mass transfer rate constant (km) was higher in flow-through mode | EC value at 4.0 and 5.0 V reached 8.6 and 23.1 Wh L−1, respectively, 5.9 and 15.9 times that of 3.0 V (1.5 Wh L−1) | [69] |
| Pd-Based REM | Simulated wastewater containing 1.0 mM NO3− | V = 0.2 and 1.8 mL min−1 U = −2.5 V/SHE | Concentration of NO3− was lower than EPAs regulatory MCL (700 μM) after a short time treating (≈2 s) | EC value of treated surface water was 1.1 to 1.3 kWh mol−1 for 1 mM NO3− | [51] |
| Ti4O7-based REM | Simulated wastewater containing 1.4 g L−1 algal cell | I = 100–500 mA t = 30–120 min | Algal cells exhibited significant disruption, while lipid extraction efficiency increased by 1.5 times for treated algae (p < 0.05) | [93] | |
| Titanium sub-oxide REM | Simulated wastewater containing ~106 CFU/mL Escherichia coli (E. coli) and ~1011 plaque forming units (PFU)/mL bacteriophage MS2 | Celectrolyte = 0.05 M Na2SO4 J = 0–10 mA cm−2 V = 5 mL min−1 | E. coli decreased from 6.46 log CFU/mL to 0.18 log CFU/mL, while bacteriophage MS2 achieved 6.74 log reduction as compared to original concentration (1011 PFU mL−1) | [94] | |
| Activated carbon fiber cloth-REM | Simulated wastewater containing 107 cellsmL−1 Escherichia coli (E. coli) | Celectrolyte = 50 mM Na2SO4 V = 1–20 mL min−1 U = 0–20 V | Disinfection was enhanced to 0.5, 1.4, 7.3, and 7.3 log reduction for the applied voltages of 2, 5, 10, and 20 V, respectively, and the log reduction of 7.3 represented complete disinfection | EC value was 1.5 kWh m−3 for a complete disinfection | [95] |
| Activated carbon fiber felt-REM | Simulated wastewater containing 106–107CFU mL−1 Escherichia coli ATCC 25922 (E. coli) | Celectrolyte = 10 mM Na2SO4 Q = 100 Lm−2 h−1 U = 2.5 V | ≈100% log removal efficiency was obtained at a low voltage of 2.5 V. Meanwhile, the system can maintain long-lasting bacterial disinfection efficiency of real wastewater (≈100% log removal) in continuous flow tests with J of 100 Lm−2 h−1 | [96] | |
| Moving-bed electrochemical membrane bioreactor (Anode as REM) | Simulated wastewater containing 100 μgL−1 sulfamethoxazole (SMX) | U = 2 V cm−1 | Removal of SMX achieved at 88.8 ± 2.4% during 91 d operation, while COD and NH4+-N removal were 93.7 ± 2.6% and > 95% | [66] | |
| β-PbO2-tubular reactive filter(TRF) | Surface water and municipal sewage treatment plant (MSTP) final effluent containing 0.5 or 0.6 mM Norfloxacin (NOR) and sulfamethoxazole (SMZ) | V = 3.57 × 10−3 m s−1 I = 0.05, 0.1–0.25 A | 90% NOR degradation were achieved with Rt of 2.0 and 3.2 s for reservoir water (0.05A) and MSTP effluent (0.25 A); Effective removal for SMZ achieved with Rt of 4.1–5.4 s | 0.005–0.024 kWh m−3 for NOR and 0.012–0.017 kWh m−3 for SMZ | [97] |
| Ti/SnO2-Sb REM | Simulated wastewater containing 20 μg L−1 stavudine | Celectrolyte = 10 mM Na2SO4 J = 2–10 mA cm−2 pH = 3.0–11.0 | Stavudine could be 100% removed by variety conditions (current density > 8 mA cm−2, pH < 5) | Ranging from 0.87 to 2.29 Wh L−1 for 90% stavudine degradation | [98] |
| Type of ECM | Treating Subject Properties | Wastewater Characteristics | Technology | Operation Conditions | Performance | Reference |
|---|---|---|---|---|---|---|
| Carbon–PTFE cathode | Leachate concentrates from a municipal landfill site | 3896 mg L−1 COD; 1347 mg L−1 TOC; 23.4 mS cm−1 conductivity pH = 7.70 | EF | Undivided reactor: 200 mL 0.4 L min−1 oxygen flow rate 1–40 mM FeSO4 J = 30 mA cm−2 pH = 2–5 | The removal efficiencies of TOC and TN were 82% and 51% within 6 h | [122] |
| Carbon–PTFE cathode | Leachate concentrate collected from a municipal landfill site (Beijing, China) | 6635 mg L−1 COD 1650 mg L−1 TOC 50.2 mS cm−1 conductivitypH = 8.07 | E-peroxone | 0.3 L min−1 O2 and O3 mixture airflow rate I = 350 mA | 87% of TOC was removed after 4 h | [123] |
| Carbon–PTFE cathode | Surface water collected from a reservoir in the suburban area of Beijing | 2.53 mg L−1 DOC 0.037 cm−1 UV254 243 uS cm−1 conductivity 4.68 mg L−1 Cl− pH = 8.03 | E-peroxone | Undivided reactor: 600 mL 150 mL min−1 O2/O3 flow rate J = 1.25–5.0 mA cm−2 | Accelerated micropollutant abatement in the surface water and all micropollutants were completely removed within 10 min | [124] |
| Carbon–PTFE air-diffusion cathode | Olive oil mill wastewater collected from a pre-Mium extra virgin olive oil production mill in northeastern Spain | 581.1 ± 2.3 mg L−1 TOC 3.50 mS cm−1 conductivity pH = 6.83 ± 0.07 | Sequential EC/PEF | Undivided reactor: 200 mL magnetic bar at 700 rpm; 0.50 mM Fe2+ J = 25 mA cm−2 pH = 3 1 L min−1 air pumped | 97.1% TOC was removed after 600 min with 115.8 kWh (kg TOC)−1 | [125] |
| Carbon–PTFE air-diffusion cathode | Real wastewater (RWW) obtained from the secondary decanter of a municipal WWTF near Barcelona | 81.1 mg L−1 total carbon 10.8 mg L−1 TOC 0.20 mg L−1 Fe2+ 2.20 mS cm−1 conductivity pH = 8.10 | Solar PEF | A 2.5L flow plant operating in batch mode 30–35 W m−2 UV irradiance | A complete removal of parabens in 180 min and 66% mineralization at 240 min. The mineralization current efficiency reported was up to 1000%, with a low energy consumption of 84 kWh (kg TOC)−1 | [126] |
| Carbon–PTFE air-diffusion cathode | Urban wastewater was collected from the secondary effluent of a wastewater treatment facility located in Gavà-Viladecans (Barcelona, Spain) | 15.0 mg L−1 TOC 318.1 mg L−1 Cl− 0.19 mg L−1 Fe2+ 3.2 mS cm−1 conductivity pH = 7.90 | PEF | An undivided, cylindrical, double-jacketed tank reactor of 150 mL 5 W m−2 UVA irradiance | 96% TOC reduction achieved in 0.050 M Na2SO4 of IrO2 based DSA® | [127] |
| Carbon–PTFE air-diffusion cathode | The raw wastewater to be spiked with synthetic food azo dyes was a secondary effluent obtained from a WWTP located in Gavá-Viladecans (Barcelona, Spain) | 15 mg L−1 DOC 66 mg L−1 TN 1.3 mM SO42− pH = 7.50 | Solar PEF | An undivided, cylindrical two-electrode glass cell with volume of 130 mL 0.50 mM Fe2+ 5 W m−2 UVA light at 360 nm | PEF-BDD is able to yielding almost total mineralization in a real water matrix (95% DOC removal) | [128] |
| Carbon–PTFE air-diffusion cathode | The secondary effluent of a WWTP located in Gavà-Viladecans (Barcelona, Spain) | 12.2 mg L−1 TOC 1.73 mS cm−1 conductivity 0.19 mg L−1 Fe2+ 318 mg L−1 Cl− 141.3 mg L−1 SO42−pH = 8.10 | PEF | Undivided cell: 150 mL 1 L min−1 air flow rate 0.05 mM Fe2+ 5 W m−2 UVA light (λmax = 360 nm) | Completely removal of tetracaine in 90 min. A 78% TOC abatement was found at 11 h, while 100% mineralization at 24 h | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, K.; Cui, T.; Huang, F.; Zhang, Y.; Han, W. Membrane Separation Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: A Short Review. Membranes 2020, 10, 337. https://doi.org/10.3390/membranes10110337
Wei K, Cui T, Huang F, Zhang Y, Han W. Membrane Separation Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: A Short Review. Membranes. 2020; 10(11):337. https://doi.org/10.3390/membranes10110337
Chicago/Turabian StyleWei, Kajia, Tao Cui, Fang Huang, Yonghao Zhang, and Weiqing Han. 2020. "Membrane Separation Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: A Short Review" Membranes 10, no. 11: 337. https://doi.org/10.3390/membranes10110337