Nanocomposite Membranes for Liquid and Gas Separations from the Perspective of Nanostructure Dimensions
Abstract
:1. Introduction
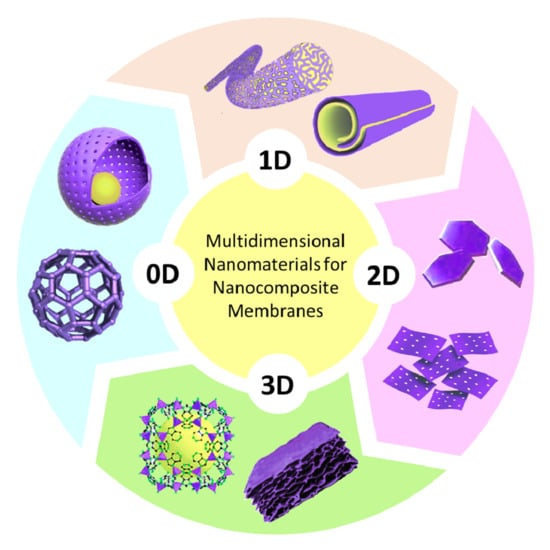
2. Dimension Plays Its Role: An Overview
2.1. Zero-Dimensional Nanomaterials
2.2. One-Dimensional Nanomaterials
2.3. Two-Dimensional Nanomaterials
2.4. Three-Dimensional Nanomaterials
3. Tailoring the Dimensions of Nanostructures to the Separation Processes
3.1. Gas Separation
3.2. Liquid Separation
4. Synergy of Multidimensional Hybrid Nanostructures
5. Anisotropy and Orientations of Nanostructures
6. Gaps and Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020, 266, 121867. [Google Scholar] [CrossRef]
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Yu, D.; Chen, X.; Cheng, R.; Min, S.; Wang, J.; Xiao, Q.; Wang, J. Overview of membrane technology applications for industrial wastewater treatment in China to increase water supply. Resour. Conserv. Recycl. 2015, 105, 1–10. [Google Scholar] [CrossRef]
- Matteucci, S.; Chakraborty, D. Risk management for industrial adoption of membrane technology. Curr. Opin. Chem. Eng. 2020, 28, 112–117. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Wang, Z.X.; Jiang, X.; Li, T.; Lau, C.H.; Guo, Z.; Ma, J.; Shao, L. Towards sustainable ultrafast molecular-separation membranes: From conventional polymers to emerging materials. Prog. Mater. Sci. 2018, 92, 258–283. [Google Scholar] [CrossRef] [Green Version]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Nasir, A.; Masood, F.; Yasin, T.; Hameed, A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019, 79, 29–40. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 343–354. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, S.-H.; Zhao, H.-L.; Wu, S.-B.; Xu, J.-M.; Li, L.; Liu, X.-Y. Layer-by-layer (LBL) assembly technology as promising strategy for tailoring pressure-driven desalination membranes. J. Membr. Sci. 2015, 493, 428–443. [Google Scholar] [CrossRef]
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R. Nanotechnology Emerging Trends, Markets, and Concerns. Nanotechnol. Saf. 2013, 1–16. [Google Scholar] [CrossRef]
- Zhang, N.; Qi, W.; Huang, L.; Jiang, E.; Bao, J.; Zhang, X.; An, B.; He, G. Review on structural control and modification of graphene oxide-based membranes in water treatment: From separation performance to robust operation. Chin. J. Chem. Eng. 2019, 27, 1348–1360. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total. Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Janakiram, S.; Ahmadi, M.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of Nanocomposite Membranes Containing 0D to 2D Nanofillers for CO2 Separation: A Review. Membranes 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, E.Y.; Toh, W.; Yeo, J.; Lin, R.; Liu, Z.; Geethalakshmi, K.; Ng, T.Y. A review on low dimensional carbon desalination and gas separation membrane designs. J. Membr. Sci. 2020, 598, 117785. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Liu, M.; Gurr, P.A.; Fu, Q.; Webley, P.; Qiao, G.G. Two-dimensional nanosheet-based gas separation membranes. J. Mater. Chem. A 2018, 6, 23169–23196. [Google Scholar] [CrossRef]
- Liu, Y. Beyond graphene oxides: Emerging 2D molecular sieve membranes for efficient separation. Chin. J. Chem. Eng. 2019, 27, 1257–1271. [Google Scholar] [CrossRef]
- Safarpour, M.; Arefi-Oskoui, S.; Khataee, A. A review on two-dimensional metal oxide and metal hydroxide nanosheets for modification of polymeric membranes. J. Ind. Eng. Chem. 2020, 82, 31–41. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, Q.; Sadighi, Z. Rational design of two-dimensional nanofillers for polymer nanocomposites toward multifunctional applications. Prog. Mater. Sci. 2021, 115, 100708. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H. Engineering Sub-Nanometer Channels in Two-Dimensional Materials for Membrane Gas Separation. Membranes 2018, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; De Adhikari, A.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ling, H.; Lamonier, J.-F.; Jaroniec, M.; Huang, J.; Monteiro, M.J.; Liu, J. A synthetic strategy for carbon nanospheres impregnated with highly monodispersed metal nanoparticles. NPG Asia Mater. 2016, 8, e240. [Google Scholar] [CrossRef] [Green Version]
- Baaziz, W.; Liu, X.; Florea, I.; Bégin-Colin, S.; Pichon, B.; Ulhaq, C.; Ersen, O.; Soria-Sánchez, M.; Zafeiratos, S.; Janowska, I.; et al. Carbon nanotube channels selectively filled with monodispersed Fe3-xO4 nanoparticles. J. Mater. Chem. A 2013, 1, 13853. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Saenz, N.; Reed, K.; Zavalij, P.; Mowery, J.D.; Bauchan, G. Facile and template-free solvothermal synthesis of mesoporous/macroporous metal–organic framework nanosheets. RSC Adv. 2018, 8, 33059–33064. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nat. Cell Biol. 2016, 539, 76–80. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Bridges, D.; Peng, P.; Lawrie, B.J.; Feng, Z.; Hu, A. Zero-dimensional to three-dimensional nanojoining: Current status and potential applications. RSC Adv. 2016, 6, 75916–75936. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Cao, W.; Mao, B.; Liu, Y.; Kang, Z. Advances in carbon dots: From the perspective of traditional quantum dots. Mater. Chem. Front. 2020, 4, 1586–1613. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef] [PubMed]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Gadgil, B.S.T.-; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C 2020, 108, 110319. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chi, H.; Zhang, C.; Cui, R.; Lu, W.; Yuan, M.; Qin, Y. Effect of multiscale structure on the gas barrier properties of poly(lactic acid)/Ag nanocomposite films. Polym. Adv. Technol. 2019, 30, 1709–1715. [Google Scholar] [CrossRef]
- Najafi, M.; Sadeghi, M.; Bolverdi, A.; Chenar, M.P.; Pakizeh, M. Gas permeation properties of cellulose acetate/silica nanocomposite membrane. Adv. Polym. Technol. 2017, 37, 2043–2052. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Ebadi Amooghin, A.; Ghanbari, D. A novel ternary mixed matrix membrane containing glycerol-modified poly(ether-block-amide) (Pebax 1657)/copper nanoparticles for CO2 separation. J. Membr. Sci. 2019, 573, 234–246. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Synthesis of a new nanocomposite membrane (PEBAX-1074/PEG-400/TiO2) in order to separate CO2 from CH4. J. Nat. Gas Sci. Eng. 2017, 37, 39–51. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Synthesis of a PEBAX-1074/ZnO nanocomposite membrane with improved CO2 separation performance. J. Energy Chem. 2017, 26, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Shirke, Y.M.; Abou-Elanwar, A.M.; Choi, W.-K.; Lee, H.; Hong, S.U.; Lee, H.K.; Jeon, J.-D. Influence of nitrogen/phosphorus-doped carbon dots on polyamide thin film membranes for water vapor/N2 mixture gas separation. RSC Adv. 2019, 9, 32121–32129. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2019, 1904362, 1–26. [Google Scholar] [CrossRef]
- Song, X.; Zhou, Q.; Zhang, T.; Xu, H.; Wang, Z. Pressure-assisted preparation of graphene oxide quantum dot-incorporated reverse osmosis membranes: Antifouling and chlorine resistance potentials. J. Mater. Chem. A 2016, 4, 16896–16905. [Google Scholar] [CrossRef]
- Zhao, D.L.; Chung, T.-S. Applications of carbon quantum dots (CQDs) in membrane technologies: A review. Water Res. 2018, 147, 43–49. [Google Scholar] [CrossRef]
- He, Y.; Zhao, D.L.; Chung, T.-S. Na+ functionalized carbon quantum dot incorporated thin-film nanocomposite membranes for selenium and arsenic removal. J. Membr. Sci. 2018, 564, 483–491. [Google Scholar] [CrossRef]
- Salestan, S.K.; Seyedpour, S.F.; Rahimpour, A.; Shamsabadi, A.A.; Tiraferri, A.; Soroush, M. Molecular Dynamics Insights into the Structural and Water Transport Properties of a Forward Osmosis Polyamide Thin-Film Nanocomposite Membrane Modified with Graphene Quantum Dots. Ind. Eng. Chem. Res. 2020, 59, 14447–14457. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef] [Green Version]
- Kalay, S.; Yilmaz, Z.; Sen, O.; Emanet, M.; Kazanc, E.; Çulha, M. Synthesis of boron nitride nanotubes and their applications. Beilstein J. Nanotechnol. 2015, 6, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron Nitride Nanotubes and Nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Kim, J.H.; Pham, T.V.; Hwang, J.H.; Kim, C.S.; Kim, M.J. Boron nitride nanotubes: Synthesis and applications. Nano Converg. 2018, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, H.; Masahiro, S.; Nodasaka, Y.; Ueda, W. Synthesis, characterization and formation process of transition metal oxide nanotubes using carbon nanofibers as templates. J. Solid State Chem. 2009, 182, 1587–1592. [Google Scholar] [CrossRef]
- Son, M.; Novotny, V.; Choi, H. Thin-film nanocomposite membrane with vertically embedded carbon nanotube for forward osmosis. Desalin. Water Treat. 2016, 57, 26670–26679. [Google Scholar] [CrossRef]
- Paineau, E. Imogolite Nanotubes: A Flexible Nanoplatform with Multipurpose Applications. Appl. Sci. 2018, 8, 1921. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Wen, X.; Kumar, U.; You, Y.; Chen, V.; Joshi, R.K. Separation and purification using GO and r-GO membranes. RSC Adv. 2018, 8, 23130–23151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Feng, Y.; Wang, F.; Yang, Z.; Wang, J. Two dimensional hexagonal boron nitride (2D-hBN): Synthesis, properties and applications. J. Mater. Chem. C 2017, 5, 11992–12022. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Van Der Bruggen, B. An MXene-based membrane for molecular separation. Environ. Sci. Nano 2020, 7, 1289–1304. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Zhou, T.; Wang, Q.; Li, Y. Recent advances in layered double hydroxides (LDHs) as two-dimensional membrane materials for gas and liquid separations. J. Membr. Sci. 2018, 567, 89–103. [Google Scholar] [CrossRef]
- Wang, X.; Chi, C.; Zhang, K.; Qian, Y.; Gupta, K.M.; Kang, Z.; Jiang, J.; Zhao, D. Reversed thermo-switchable molecular sieving membranes composed of two-dimensional metal-organic nanosheets for gas separation. Nat. Commun. 2017, 8, 14460. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.; Xu, M.; Jiang, B.; Wen, G.; Zhao, L.; Wang, B.; Yu, A.; Bai, Z.; Sun, Y.; Zhang, L.; et al. Bioinspired Graphene Oxide Membranes with Dual Transport Mechanisms for Precise Molecular Separation. Adv. Funct. Mater. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Guan, G.; Han, M. Functionalized Hybridization of 2D Nanomaterials. Adv. Sci. 2019, 6, 1901837. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Zhang, B.; Gu, Y.; Yu, M.; Wang, D.; Wu, J.; Li, J. Tailored Graphene Oxide Membranes for the Separation of Ions and Molecules. ACS Appl. Nano Mater. 2019, 2, 6611–6621. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Gao, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, X.; Yang, X.; Bai, Y.; Shao, L. Nanoporous framework “reservoir” maximizing low-molecular-weight enhancer impregnation into CO2-philic membranes for highly-efficient CO2 capture. J. Membr. Sci. 2019, 278–285. [Google Scholar] [CrossRef]
- Yang, S.; Karve, V.V.; Justin, A.; Kochetygov, I.; Espín, J.; Asgari, M.; Trukhina, O.; Sun, D.T.; Peng, L.; Queen, W.L. Enhancing MOF performance through the introduction of polymer guests. Coord. Chem. Rev. 2021, 427, 213525. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Kosinov, N.N.; Gascon, J.; Kapteijn, F.; Hensen, E.J. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Krachuamram, S.; Chanapattharapol, K.C.; Kamonsutthipaijit, N. Synthesis and characterization of NaX-type zeolites prepared by different silica and alumina sources and their CO2 adsorption properties. Microporous Mesoporous Mater. 2021, 310, 110632. [Google Scholar] [CrossRef]
- Borjigin, B.; Liu, L.; Yu, L.; Xu, L.; Zhao, C.; Wang, J. Influence of incorporating beta zeolite nanoparticles on water permeability and ion selectivity of polyamide nanofiltration membranes. J. Environ. Sci. 2020, 98, 77–84. [Google Scholar] [CrossRef]
- Dai, Q.; Lu, W.; Zhao, Y.; Zhang, H.; Zhu, X.; Li, X. Advanced scalable zeolite “ions-sieving” composite membranes with high selectivity. J. Membr. Sci. 2020, 595, 117569. [Google Scholar] [CrossRef]
- Přech, J.; Pizarro, P.; Serrano, D.P.; Čejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, M.; Zhao, H.; Jiang, Y.; Liu, G.; Gao, J. Enhanced dispersibility of metal–organic frameworks (MOFs) in the organic phase via surface modification for TFN nanofiltration membrane preparation. RSC Adv. 2020, 10, 4045–4057. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, H. Zeolitic imidazolate framework composite membranes and thin films: Synthesis and applications. Chem. Soc. Rev. 2014, 43, 4470–4493. [Google Scholar] [CrossRef]
- Wang, T.; Lin, E.; Peng, Y.-L.; Chen, Y.; Cheng, P.; Zhang, Z. Rational design and synthesis of ultramicroporous metal-organic frameworks for gas separation. Coord. Chem. Rev. 2020, 423, 213485. [Google Scholar] [CrossRef]
- Li, M.-P.; Zhang, X.; Zhang, H.; Liu, W.-L.; Huang, Z.-H.; Xie, F.; Ma, X.-H.; Xu, Z.-L. Hydrophilic yolk-shell ZIF-8 modified polyamide thin-film nanocomposite membrane with improved permeability and selectivity. Sep. Purif. Technol. 2020, 247, 116990. [Google Scholar] [CrossRef]
- Beh, J.; Ooi, B.; Lim, J.; Ng, E.; Mustapa, H. Development of high water permeability and chemically stable thin film nanocomposite (TFN) forward osmosis (FO) membrane with poly(sodium 4-styrenesulfonate) (PSS)-coated zeolitic imidazolate framework-8 (ZIF-8) for produced water treatment. J. Water Process. Eng. 2020, 33, 101031. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Membr. Sci. 2019, 23–33. [Google Scholar] [CrossRef]
- Japip, S.; Erifin, S.; Chung, T.-S. Reduced thermal rearrangement temperature via formation of zeolitic imidazolate framework (ZIF)-8-based nanocomposites for hydrogen purification. Sep. Purif. Technol. 2019, 212, 965–973. [Google Scholar] [CrossRef]
- Zheng, W.; Ding, R.; Yang, K.; Dai, Y.; Yan, X.; He, G. ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs. Sep. Purif. Technol. 2019, 214, 111–119. [Google Scholar] [CrossRef]
- Zheng, W.; Tsang, C.-S.; Lee, L.Y.S.; Wong, K.-Y. Two-dimensional metal-organic framework and covalent-organic framework: Synthesis and their energy-related applications. Mater. Today Chem. 2019, 12, 34–60. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-Flux Membranes Based on the Covalent Organic Framework COF-LZU1 for Selective Dye Separation by Nanofiltration. Angew. Chem. Int. Ed. 2018, 57, 4083–4087. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van Der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, J.; Shan, B.; Wang, X.; Gao, C. TpPa-2-incorporated mixed matrix membranes for efficient water purification. J. Membr. Sci. 2017, 526, 355–366. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Tian, L.; Zhang, J.; Su, B.; Hu, M.Z. Covalent organic frameworks (COFs)-incorporated thin film nanocomposite (TFN) membranes for high-flux organic solvent nanofiltration (OSN). J. Membr. Sci. 2019, 572, 520–531. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Bae, T.-H.; Yang, Y.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Melamine-based covalent organic framework-incorporated thin film nanocomposite membrane for enhanced osmotic power generation. Desalination 2019, 459, 10–19. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS Polymers: Physical Properties and Biomaterials Applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Caro, J. Are MOF membranes better in gas separation than those made of zeolites? Curr. Opin. Chem. Eng. 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Wu, T.; Prasetya, N.; Li, K. Recent advances in aluminium-based metal-organic frameworks (MOF) and its membrane applications. J. Membr. Sci. 2020, 615, 118493. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, S.; Wang, J.; Zhang, Y.; Tian, M.; Van Der Bruggen, B. Microporous organic polymer-based membranes for ultrafast molecular separations. Prog. Polym. Sci. 2020, 110, 101308. [Google Scholar] [CrossRef]
- Rezaei-DashtArzhandi, M.; Ismail, A.; Goh, P.S.; Azelee, I.W.; Abbasgholipourghadim, M.; Rehman, G.U.; Matsuura, T. Zeolite ZSM5-Filled PVDF Hollow Fiber Mixed Matrix Membranes for Efficient Carbon Dioxide Removal via Membrane Contactor. Ind. Eng. Chem. Res. 2016, 55, 12632–12643. [Google Scholar] [CrossRef]
- Lee, P.S.; Hong, D.-Y.; Cha, G.-Y.; An, H.; Moon, S.-Y.; Seong, M.; Chang, B.-J.; Lee, J.S.; Kim, J.-H. Mixed matrix membranes incorporated with three-dimensionally ordered mesopore imprinted (3DOm-i) zeolite. Sep. Purif. Technol. 2019, 210, 29–37. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, K.; Liu, L.; Liu, M.; Qiu, W.; Webley, P.A. Enhancing plasticization-resistance of mixed-matrix membranes with exceptionally high CO2/CH4 selectivity through incorporating ZSM-25 zeolite. J. Membr. Sci. 2019, 583, 23–30. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Ozen, H.A.; Ozturk, B. Gas separation characteristic of mixed matrix membrane prepared by MOF-5 including different metals. Sep. Purif. Technol. 2019, 211, 514–521. [Google Scholar] [CrossRef]
- Akbari, A.; Karimi-Sabet, J.; Ghoreishi, S.M. Matrimid® 5218 based mixed matrix membranes containing metal organic frameworks (MOFs) for helium separation. Chem. Eng. Process. Process. Intensif. 2020, 148, 107804. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Wakimoto, K.; Gibbons, A.H.; Isfahani, A.P.; Kusuda, H.; Sivaniah, E.; Ghalei, B. Enhanced PIM-1 membrane gas separation selectivity through efficient dispersion of functionalized POSS fillers. J. Membr. Sci. 2017, 539, 178–186. [Google Scholar] [CrossRef]
- Guerrero, G.; Hägg, M.B.; Kignelman, G.; Simon, C.; Peters, T.; Rival, N.; Denonville, C. Investigation of amino and amidino functionalized Polyhedral Oligomeric SilSesquioxanes (POSS®) nanoparticles in PVA-based hybrid membranes for CO2/N2 separation. J. Membr. Sci. 2017, 544, 161–173. [Google Scholar] [CrossRef]
- Konnertz, N.; Ding, Y.; Harrison, W.J.; Budd, P.M.; Schönhals, A.; Böhning, M. Molecular mobility and gas transport properties of nanocomposites based on PIM-1 and polyhedral oligomeric phenethyl-silsesquioxanes (POSS). J. Membr. Sci. 2017, 529, 274–285. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total. Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Safarifard, V. Carbon dioxide capture in MOFs: The effect of ligand functionalization. Polyhedron 2018, 154, 236–251. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Kwon, S.; Goh, K.; Wang, R.; Na, K.; Bae, T.-H. Nanosizing zeolite 5A fillers in mixed-matrix carbon molecular sieve membranes to improve gas separation performance. Chem. Eng. J. Adv. 2020, 2, 100016. [Google Scholar] [CrossRef]
- Ishaq, S.; Tamime, R.; Bilad, M.R.; Khan, A.L. Mixed matrix membranes comprising of polysulfone and microporous Bio-MOF-1: Preparation and gas separation properties. Sep. Purif. Technol. 2019, 210, 442–451. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Hou, L.; Yang, C.; Shen, H.; Yang, K.; Wang, Z. Metal-organic framework MOF-801/PIM-1 mixed-matrix membranes for enhanced CO2/N2 separation performance. Sep. Purif. Technol. 2020, 250, 117198. [Google Scholar] [CrossRef]
- Khosravi, A.; Vatani, A.; Mohammadi, T. Application of polyhedral oligomeric silsesquioxane to the stabilization and performance enhancement of poly(4-methyl-2-pentyne) nanocomposite membranes for natural gas conditioning. J. Appl. Polym. Sci. 2017, 134, 45158. [Google Scholar] [CrossRef]
- Iyer, P.; Iyer, G.; Coleman, M.R. Gas transport properties of polyimide–POSS nanocomposites. J. Membr. Sci. 2010, 358, 26–32. [Google Scholar] [CrossRef]
- Akbari, A.; Karimi-Sabet, J.; Ghoreishi, S.M. Polyimide based mixed matrix membranes incorporating Cu-BDC nanosheets for impressive helium separation. Sep. Purif. Technol. 2020, 253, 117430. [Google Scholar] [CrossRef]
- Shete, M.; Kumar, P.; Bachman, J.E.; Ma, X.; Smith, Z.P.; Xu, W.; Mkhoyan, K.A.; Long, J.R.; Tsapatsis, M. On the direct synthesis of Cu(BDC) MOF nanosheets and their performance in mixed matrix membranes. J. Membr. Sci. 2018, 549, 312–320. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.; Gottmer, S.; Chu, L.; Gascon, J.; Kapteijn, F. Thin mixed matrix and dual layer membranes containing metal-organic framework nanosheets and Polyactive™ for CO2 capture. J. Membr. Sci. 2019, 226–235. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Hu, Z.; Qian, Y.; Chi, C.; Yeo, L.Y.; Tee, L.; Zhao, D. Mixed matrix membranes composed of two-dimensional metal–organic framework nanosheets for pre-combustion CO2 capture: A relationship study of filler morphology versus membrane performance. J. Mater. Chem. A 2015, 3, 20801–20810. [Google Scholar] [CrossRef]
- Yinab, H.; Alkasa, A.; Zhanga, Y.; Zhangc, Y.; Telfer, S.G. Mixed matrix membranes (MMMs) using an emerging metal-organic framework (MUF-15) for CO2 separation. J. Membr. Sci. 2020, 609, 118245. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Xamena, F.X.L.I.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2014, 14, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Ji, Y.; Liu, Q.; Cheng, L.; Guan, K.; Zhang, M.; Liu, G.; Xiong, J.; Yang, J.; et al. 2D MXene Nanofilms with Tunable Gas Transport Channels. Adv. Funct. Mater. 2018, 28, 1–13. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. 2D Metal–Organic Frameworks as Multifunctional Materials in Heterogeneous Catalysis and Electro/Photocatalysis. Adv. Mater. 2019, 31, e1900617. [Google Scholar] [CrossRef]
- Janakiram, S.; Espejo, J.L.M.; Yu, X.; Ansaloni, L.; Deng, L. Facilitated transport membranes containing graphene oxide-based nanoplatelets for CO2 separation: Effect of 2D filler properties. J. Membr. Sci. 2020, 616, 118626. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Agrawal, K.V.; Coronas, J. Ultrathin permselective membranes: The latent way for efficient gas separation. RSC Adv. 2020, 10, 12653–12670. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ismail, A. Thin film nanocomposite: The next generation selective membrane for CO 2 removal. J. Mater. Chem. A 2016, 4, 15726–15748. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Li, X.; Wang, Z.; Tang, C.Y. Metal-Organic Framework Nanosheets for Thin-Film Composite Membranes with Enhanced Permeability and Selectivity. ACS Appl. Nano Mater. 2020, 3, 9238–9248. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Saraswat, V.; Jacobberger, R.M.; Ostrander, J.S.; Hummell, C.L.; Way, A.J.; Arnold, M.S.; Zanni, M.T.; Arnold, M.S. Invariance of Water Permeance through Size-Differentiated Graphene Oxide Laminates. ACS Nano 2018, 12, 7855–7865. [Google Scholar] [CrossRef]
- Nie, L.; Goh, K.; Wang, Y.; Lee, J.; Huang, Y.; Karahan, H.E.; Zhou, K.; Guiver, M.D.; Bae, T.-H. Realizing small-flake graphene oxide membranes for ultrafast size-dependent organic solvent nanofiltration. Sci. Adv. 2020, 6, eaaz9184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, A.; Bozorg, A.; Shakeri, A. Novel Dimensionally Controlled Nanopore Forming Template in Forward Osmosis Membranes. Environ. Sci. Technol. 2018, 52, 2704–2716. [Google Scholar] [CrossRef]
- Kellenberger, C.; Luechinger, N.A.; Lamprou, A.; Rossier, M.; Grass, R.N.; Stark, W.J. Soluble nanoparticles as removable pore templates for the preparation of polymer ultrafiltration membranes. J. Membr. Sci. 2012, 387, 76–82. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Li, X.; Liu, Y.; Xie, J. Template-Assisted Fabrication of Thin-Film Composite Forward-Osmosis Membrane with Controllable Internal Concentration Polarization. Ind. Eng. Chem. Res. 2016, 55, 5327–5334. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Yang, S.; Wei, Y.; Zhang, Z.; Li, Y.-S. Layered double hydroxides (LDHs) as novel macropore-templates: The importance of porous structures for forward osmosis desalination. J. Membr. Sci. 2019, 585, 175–183. [Google Scholar] [CrossRef]
- Huangfu, X.; Xu, Y.; Liu, C.; He, Q.; Ma, J.; Ma, C.; Huang, R. A review on the interactions between engineered nanoparticles with extracellular and intracellular polymeric substances from wastewater treatment aggregates. Chemosphere 2019, 219, 766–783. [Google Scholar] [CrossRef]
- Nasir, A.M.; Goh, P.; Abdullah, M.S.; Ng, B.C.; Ismail, A.; Cheer, N.B. Adsorptive nanocomposite membranes for heavy metal remediation: Recent progresses and challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef]
- Diab, M.; Attia, N.F.; Attia, A.; El-Shahat, M. Green Synthesis of Cost-Effective and Efficient Nanoadsorbents Based on Zero and Two Dimensional Nanomaterials for Zn2+ and Cr3+ Removal from Aqueous Solutions. Synth. Met. 2020, 265, 116411. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube / polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Al Aani, S.; Gomez, V.; Wright, C.J.; Hilal, N. Fabrication of antibacterial mixed matrix nanocomposite membranes using hybrid nanostructure of silver coated multi-walled carbon nanotubes. Chem. Eng. J. 2017, 326, 721–736. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Verma, K.; Patyal, A.; Sharma, I.; Barman, P.; Sharma, D. Nanosheet and nanosphere morphology dominated photocatalytic & antibacterial properties of ZnO nanostructures. Solid State Sci. 2019, 89, 1–14. [Google Scholar] [CrossRef]
- Ananth, A.; Dharaneedharan, S.; Gandhi, M.S.; Heo, M.-S.; Mok, Y.S. Novel RuO2 nanosheets–Facile synthesis, characterization and application. Chem. Eng. J. 2013, 223, 729–736. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Yinab, J.; Zhanb, F.; Jiao, T.; Dengb, H.; Zoua, G.; Baic, Z.; Zhangb, Q.; Peng, Q. Highly efficient catalytic performances of nitro compounds via hierarchical PdNPs-loaded MXene/polymer nanocomposites synthesized through electrospinning strategy for wastewater treatment. Chin. Chem. Lett. 2020, 31, 992–995. [Google Scholar] [CrossRef]
- Yanga, W.; Hua, W.; Zhanga, J.; Wanga, W.; Caib, R.; Pana, M.; Huanga, C.; Chenb, X.; Yanc, B.; Zenga, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.; Yang, J.; Wang, B. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10. [Google Scholar] [CrossRef]
- Ahmad, N.; Noh, A.M.; Leo, C.; Ahmad, A. CO2 removal using membrane gas absorption with PVDF membrane incorporated with POSS and SAPO-34 zeolite. Chem. Eng. Res. Des. 2017, 118, 238–247. [Google Scholar] [CrossRef]
- Galve, A.; Sieffert, D.; Staudt, C.; Ferrando, M.; Güell, C.; Téllez, C.; Coronas, J. Combination of ordered mesoporous silica MCM-41 and layered titanosilicate JDF-L1 fillers for 6FDA-based copolyimide mixed matrix membranes. J. Membr. Sci. 2013, 431, 163–170. [Google Scholar] [CrossRef]
- Jamil, N.; Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Roslan, R.A.; Lau, W.J.; Ismail, A.F. Mixed matrix membranes incorporated with reduced graphene oxide (rGO) and zeolitic imidazole framework-8 (ZIF-8) nanofillers for gas separation. J. Solid State Chem. 2019, 270, 419–427. [Google Scholar] [CrossRef]
- Lia, X.; Yua, S.; Lia, K.; Mab, C.; Zhanga, J.; Lia, H.; Changa, X.; Zhua, L.; Xuea, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248, 117080. [Google Scholar] [CrossRef]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Membr. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.; Taniguchi, T.; Ismail, A.F.; Zahri, K. The role of geometrically different carbon-based fillers on the formation and gas separation performance of nanocomposite membranes. Carbon 2019, 149, 33–44. [Google Scholar] [CrossRef]
- Shi, F.; Sun, J.; Wang, J.; Liu, M.; Wang, S.; Cao, X.; Yan, Z.; Li, Y.; Nunes, S.P. Exploration of the Synergy Between 2D Nanosheets and a Non-2D Filler in Mixed Matrix Membranes for Gas Separation. Front. Chem. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Sivakumar, M.; Liu, D.-K.; Chiao, Y.-H.; Hung, W.-S. Synergistic effect of one-dimensional silk nanofiber and two-dimensional graphene oxide composite membrane for enhanced water purification. J. Membr. Sci. 2020, 606, 118142. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, H.; Bai, J.; Liu, J.; Zhang, Y. A porous graphene composite membrane intercalated by halloysite nanotubes for efficient dye desalination. Desalination 2017, 420, 145–157. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Gao, X.; Zheng, J.; Wang, J.; Volodin, A.; Xie, Y.F.; Huang, X.; Van Der Bruggen, B.; Zhu, J. High-performance thin film nanocomposite membranes enabled by nanomaterials with different dimensions for nanofiltration. J. Membr. Sci. 2020, 596, 117717. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.; Zhang, S.; Si, X.; Bai, Y.; Zhang, C. NH2-Fe3O4-regulated graphene oxide membranes with well-defined laminar nanochannels for desalination of dye solutions. Desalination 2020, 476, 114227. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van Der Bruggen, B. Construction of TiO2 @graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, G.; Han, K.; Ye, H.; Wei, S.; Zhou, Y. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process. Process. Intensif. 2017, 120, 20–26. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Khodi, S.; Xu, X.; Agathopoulos, S. Multifunctional Thin-Film Nanofiltration Membrane Incorporated with Reduced Graphene Oxide@TiO2@Ag Nanocomposites for High Desalination Performance, Dye Retention, and Antibacterial Properties. ACS Appl. Mater. Interfaces 2019, 11, 23535–23545. [Google Scholar] [CrossRef] [PubMed]
- Dadvar, E.; Kalantary, R.R.; Panahi, H.A.; Peyravi, M. Efficiency of Polymeric Membrane Graphene Oxide-TiO2for Removal of Azo Dye. J. Chem. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Peng, G.; Liao, J.; Shen, J.; Gao, C. Preparation of molecular selective GO/DTiO2-PDA-PEI composite nanofiltration membrane for highly pure dye separation. J. Membr. Sci. 2020, 601, 601. [Google Scholar] [CrossRef]
- Chung, Y.T.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Johnson, D.; Hilal, N. Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 2017, 402, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, N.; Wen, Z.; Zhang, L.; Chen, Q.; Chen, L.; Si, P.; Feng, J.; Li, Y.; Lou, J.; et al. Graphene oxide based membrane intercalated by nanoparticles for high performance nanofiltration application. Chem. Eng. J. 2018, 347, 12–18. [Google Scholar] [CrossRef]
- Liu, G.-F.; Huang, L.-J.; Wang, Y.-X.; Tang, J.; Wang, Y.; Cheng, M.-M.; Zhang, Y.; Kipper, M.J.; Belfiore, L.A.; Ranil, W.S. Preparation of a graphene/silver hybrid membrane as a new nanofiltration membrane. RSC Adv. 2017, 7, 49159–49165. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Liang, S.; Zhang, S.; Xiao, K.; Wang, X.; Li, M.; Huang, X. Surface functionalization via synergistic grafting of surface-modified silica nanoparticles and layered double hydroxide nanosheets for fabrication of superhydrophilic but relatively oleophobic antifouling membranes. Sep. Purif. Technol. 2020, 247, 116955. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J.R. Efficient separation of biological macromolecular proteins by polyethersulfone hollow fiber ultrafiltration membranes modified with Fe3O4 nanoparticles-decorated carboxylated graphene oxide nanosheets. Int. J. Biol. Macromol. 2019, 135, 798–807. [Google Scholar] [CrossRef]
- Lee, T.H.; Roh, J.S.; Yoo, S.Y.; Roh, J.M.; Choi, T.H.; Park, H.B. High-Performance Polyamide Thin-Film Nanocomposite Membranes Containing ZIF-8/CNT Hybrid Nanofillers for Reverse Osmosis Desalination. Ind. Eng. Chem. Res. 2020, 59, 5324–5332. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Ye, Z.; Yang, X.; Chen, X.; Ma, J.; Li, F. Novel Halloysite Nanotubes Intercalated Graphene Oxide Based Composite Membranes for Multifunctional Applications: Oil/Water Separation and Dyes Removal. Ind. Eng. Chem. Res. 2017, 56, 10472–10481. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef]
- Gao, S.J.; Qin, H.; Liu, P.; Jin, J. SWCNT-intercalated GO ultrathin films for ultrafast separation of molecules. J. Mater. Chem. A 2015, 3, 6649–6654. [Google Scholar] [CrossRef]
- Zengab, H.; YuABC, Z.; Shaoab, L.; Liab, X.; Zhuab, M.; Liuab, Y.; Fengab, X.; Zhuab, X. Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(VI) and dyes based on filtration. Desalination 2020, 491, 114558. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Fang, Q.; Zuo, K.; Hou, G.; Ai, Q.; Li, Q.; Ci, L.; Lou, J. High performance hierarchically nanostructured graphene oxide/covalent organic framework hybrid membranes for stable organic solvent nanofiltration. Appl. Mater. Today 2020, 20, 100791. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- Khan, N.A.; Yuan, J.; Wu, H.; Cao, L.; Zhang, R.; Liu, Y.-N.; Li, L.; Rahman, A.U.; Kasher, R.; Jiang, Z. Mixed Nanosheet Membranes Assembled from Chemically Grafted Graphene Oxide and Covalent Organic Frameworks for Ultra-high Water Flux. ACS Appl. Mater. Interfaces 2019, 11, 28978–28986. [Google Scholar] [CrossRef]
- Song, N.; Sun, Y.; Xie, X.; Wang, D.; Shao, F.; Yu, L.; Dong, J. Doping MIL-101(Cr)@GO in polyamide nanocomposite membranes with improved water flux. Desalination 2020, 492, 114601. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Shao, L.; Li, X.; Zhu, M.; Liu, Y.; Feng, X.; Zhu, X. A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021, 403, 126281. [Google Scholar] [CrossRef]
- Guan, K.; Zhao, D.; Zhang, M.; Shen, J.; Zhou, G.; Liu, G.; Jin, W. 3D nanoporous crystals enabled 2D channels in graphene membrane with enhanced water purification performance. J. Membr. Sci. 2017, 542, 41–51. [Google Scholar] [CrossRef]
- Su, B.; Wu, Y.; Jiang, L. The art of aligning one-dimensional (1D) nanostructures. Chem. Soc. Rev. 2012, 41, 7832–7856. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.; Ismail, A.; Ng, B. Directional alignment of carbon nanotubes in polymer matrices: Contemporary approaches and future advances. Compos. Part A Appl. Sci. Manuf. 2014, 56, 103–126. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-selective PEO-PBT (PolyActiveTM)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Shen, Q.; Hou, J.; Sutrisna, P.D.; Chen, V. Shear-aligned graphene oxide laminate/Pebax ultrathin composite hollow fiber membranes using a facile dip-coating approach. J. Mater. Chem. A 2017, 5, 7732–7737. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Zhu, L.; Cui, F.; Xie, C.; Chen, X.; Li, N. High-performance nanofiltration membrane with structurally controlled PES substrate containing electrically aligned CNTs. J. Membr. Sci. 2020, 605, 118104. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, Y.; Liu, J.-D.; Hou, J.; Zhang, Y.; Wang, J.; Zhu, J.; Zhang, B.; Lvov, Y.; Van Der Bruggen, B. Oriented Clay Nanotube Membrane Assembled on Microporous Polymeric Substrates. ACS Appl. Mater. Interfaces 2016, 8, 34914–34923. [Google Scholar] [CrossRef]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned Multiwalled Carbon Nanotube Membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Samieirad, S.; Mousavi, S.M.; Saljoughi, E. Alignment of functionalized multiwalled carbon nanotubes in forward osmosis membrane support layer induced by electric and magnetic fields. Powder Technol. 2020, 364, 538–552. [Google Scholar] [CrossRef]
- Li, M.; Brant, J.A. Effects of aluminogermanate imogolite nanotube orientation on mass transport across polyamide nanocomposite membranes. J. Membr. Sci. 2019, 585, 38–51. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J. Membr. Sci. 2019, 572, 38–60. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nat. Cell Biol. 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.-Z.; Zhao, L.; Wan, Y.-J.; Tang, L.-C. Three-dimensional graphene-based polymer nanocomposites: Preparation, properties and applications. Nanoscale 2018, 10, 14788–14811. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Kyotani, T. Zeolite-templated carbons–three-dimensional microporous graphene frameworks. Chem. Commun. 2018, 54, 5648–5673. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Ding, H.; Yuan, Z.; Leong, C.F.; Goh, K.; Li, W.; Yang, N.; D’Alessandro, D.M.; Chen, Y. The roles of metal-organic frameworks in modulating water permeability of graphene oxide-based carbon membranes. Carbon 2019, 148, 277–289. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, P.S.; Wong, K.C.; Ismail, A.F. Nanocomposite Membranes for Liquid and Gas Separations from the Perspective of Nanostructure Dimensions. Membranes 2020, 10, 297. https://doi.org/10.3390/membranes10100297
Goh PS, Wong KC, Ismail AF. Nanocomposite Membranes for Liquid and Gas Separations from the Perspective of Nanostructure Dimensions. Membranes. 2020; 10(10):297. https://doi.org/10.3390/membranes10100297
Chicago/Turabian StyleGoh, Pei Sean, Kar Chun Wong, and Ahmad Fauzi Ismail. 2020. "Nanocomposite Membranes for Liquid and Gas Separations from the Perspective of Nanostructure Dimensions" Membranes 10, no. 10: 297. https://doi.org/10.3390/membranes10100297