Separation of Fructose and Glucose via Nanofiltration in Presence of Fructooligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Filtration Experiment Setup
2.3. Analyses
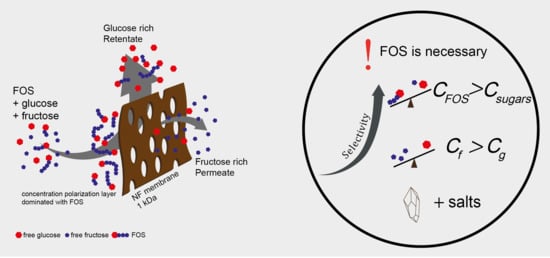
3. Results and Discussion
3.1. The Effect of Oligosaccharides
3.2. Effect of Monosaccharides Composition
3.3. Effect of Electrolytes
3.4. General Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hobb, L. Sweeteners from Starch: Production, Properties and Uses. In Starch; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 797–832. [Google Scholar] [CrossRef]
- Riby, J.E.; Fujisawa, T.; Kretchmer, N. Fructose absorption. Am. J. Clin. Nutr. 1993, 58, 748S–753S. [Google Scholar] [CrossRef] [PubMed]
- Bantle, J.P. Dietary Fructose and Metabolic Syndrome and Diabetes. J. Nutr. 2009, 139, 1263S–1268S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.M.; Conforti, F.D. Fructose. In Encyclopedia of Food Sciences and Nutrition; Elsevier B.V.: Amsterdam, The Netherlands, 2003; pp. 2748–2752. [Google Scholar]
- Pigman, W.W. Occurence, properties, synthesis and analysis of the monosaccharides. In Chemistry of the Carbohydrates; Elsevier: Amsterdam, The Netherlands, 1948; pp. 89–148. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Martinez, K.C.L.; Brito, A.B.N.; Giulietti, M. Separation of glucose and fructose by freezing crystallization. Cryst. Res. Technol. 2010, 45, 1032–1034. [Google Scholar] [CrossRef]
- Mostafazadeh, A.K.; Sarshar, M.; Javadian, S.; Zarefard, M.R.; Haghighi, Z.A. Separation of fructose and glucose from date syrup using resin chromatographic method: Experimental data and mathematical modeling. Sep. Purif. Technol. 2011, 79, 72–78. [Google Scholar] [CrossRef]
- Robards, K.; Whitelaw, M. Chromatography of monosaccharides and disaccharides. J. Chromatogr. A 1986, 373, 81–110. [Google Scholar] [CrossRef]
- Viard, V.; Lameloise, M.L. Modelling glucose-fructose separation by adsorption chromatography on ion exchange resins. J. Food Eng. 1992, 17, 29–48. [Google Scholar] [CrossRef]
- Di Luccio, M.; Smith, B.D.; Kida, T.; Borges, C.P.; Alves, T.L.M. Separation of fructose from a mixture of sugars using supported liquid membranes. J. Memb. Sci. 2000, 174, 217–224. [Google Scholar] [CrossRef]
- Almazán, J.E.; Romero-Dondiz, E.M.; Rajal, V.B.; Castro-Vidaurre, E.F. Nanofiltration of glucose: Analysis of parameters and membrane characterization. Chem. Eng. Res. Des. 2015, 94, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Hinkova, A.; Bubník, Z.; Kadlec, P.; Pridal, J. Potentials of separation membranes in the sugar industry. Sep. Purif. Technol. 2002, 26, 101–110. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Trevisan, S.; Pimentel-Souza, J.D.R.; Pastore, G.M.; Hubinger, M.D. Clarification and concentration of oligosaccharides from artichoke extract by a sequential process with microfiltration and nanofiltration membranes. J. Food Eng. 2016, 180, 120–128. [Google Scholar] [CrossRef]
- Goulas, A.K.; Kapasakalidis, P.G.; Sinclair, H.R.; Rastall, R.A.; Grandison, A.S. Purification of oligosaccharides by nanofiltration. J. Memb. Sci. 2002, 209, 321–335. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Filho, F.M.; Silva, V.; Palacio, L.; Hernández, A.; Prádanos, P. Mass transfer and transport during purification of fructooligosaccharides by nanofiltration. J. Memb. Sci. 2010, 365, 356–365. [Google Scholar] [CrossRef]
- Montesdeoca, V.A.; Bakker, J.; Boom, R.M.; Janssen, A.E.M.; Van der Padt, A. Ultrafiltration of non-spherical molecules. J. Memb. Sci. 2019, 570–571, 322–332. [Google Scholar] [CrossRef]
- Rizki, Z.; Janssen, A.E.M.; Boom, R.M.; van der Padt, A. Oligosaccharides fractionation cascades with 3 outlet streams. Sep. Purif. Technol. 2019, 221, 183–194. [Google Scholar] [CrossRef]
- Alles, M.J.L.; Tessaro, I.C.; Noreña, C.P.Z. Concentration and Purification of Yacon (Smallanthus sonchifolius) Root Fructooligosaccharides Using Membrane Technology. Food Technol. Biotechnol. 2015, 53, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Bandini, S.; Morelli, V. Effect of temperature, pH and composition on nanofiltration of mono/disaccharides: Experiments and modeling assessment. J. Memb. Sci. 2017, 533, 57–74. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Modelling the performance of membrane nanofiltration-critical assessment and model development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Montesdeoca, V.A.; Van der Padt, A.; Boom, R.M.; Janssen, A.E.M. Modelling of membrane cascades for the purification of oligosaccharides. J. Memb. Sci. 2016, 520, 712–722. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Chen, T.; Chen, C. Study on nanofiltration for purifying fructo-oligosaccharides I. Operation modes. J. Memb. Sci. 2004, 245, 123–129. [Google Scholar] [CrossRef]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and oligofructose as dietary fiber: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87 (Suppl. 2), S287–S291. [Google Scholar] [CrossRef] [Green Version]
- Jaseja, M.; Perlin, A.S.; Dais, P. Two-dimensional NMR spectral study of the tautomeric equilibria of D-fructose and related compounds. Magn. Reson. Chem. 1990, 28, 283–289. [Google Scholar] [CrossRef]
- Maple, S.R.; Allerhand, A. Detailed Tautomeric Equilibrium of Aqueous D-Glucose. Observation of Six Tautomers by Ultrahigh Resolution Carbon-13 NMR. J. Am. Chem. Soc. 1987, 109, 3168–3169. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.; Petrovsky, N. Observation of the keto tautomer of d-fructose in D 2O using 1H NMR spectroscopy. Carbohydr. Res. 2012, 347, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajmir-Riahi, H.A. Sugar interaction with calcium ion. synthesis and vibrational spectra of crystalline β-d-fructose and its calcium halide adducts. J. Inorg. Biochem. 1986, 27, 123–131. [Google Scholar] [CrossRef]
- Rao, C.P.; Geetha, K.; Raghavan, M.S.S. Fe(III) complexes of D-glucose and D-fructose. BioMetals 1994. [Google Scholar] [CrossRef]
- Angyal, S.J. Complex formation between sugars and metal ions. In Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 1973; pp. 131–146. [Google Scholar] [CrossRef]
- Kim, S.S.; Chang, H.N.; Ghim, Y.S. Separation of Fructose and Glucose by Reverse Osmosis. Ind. Eng. Chem. Fundam. 1985, 24, 409–412. [Google Scholar] [CrossRef]
- Rizki, Z.; Suryawirawan, E.; Janssen, A.E.M.; van der Padt, A.; Boom, R.M. Modelling temperature effects in a membrane cascade system for oligosaccharides. J. Memb. Sci. 2020, 118292. [Google Scholar] [CrossRef]
- Bruni, F.; Di Mino, C.; Imberti, S.; McLain, S.E.; Rhys, N.H.; Ricci, M.A. Hydrogen Bond Length as a Key to Understanding Sweetness. J. Phys. Chem. Lett. 2018, 9, 3667–3672. [Google Scholar] [CrossRef]
- Imberti, S.; McLain, S.E.; Rhys, N.H.; Bruni, F.; Ricci, M.A. Role of Water in Sucrose, Lactose, and Sucralose Taste: The Sweeter, the Wetter? ACS Omega 2019, 4, 22392–22398. [Google Scholar] [CrossRef] [Green Version]






| Component | Concentration (wt %) a |
|---|---|
| Glucose | 6.9 ± 0.5 |
| Fructose | 2.0 ± 0.2 |
| DP2 | 10.0 ± 1.1 |
| DP3 | 23.6 ± 1.5 |
| DP4 | 23.9 ± 1.4 |
| DP5 and higher | 33.6 ± 2.3 |
| Experiments | CDP1 | CDP ≥ 3 | CDP1/CDP ≥ 1 | Cfru/Cglu | CNa+ or CCa2+ |
|---|---|---|---|---|---|
| (g/L) | (g/L) | (g/L) | |||
| Experiment A | 9–140 | 35 | 0.25–4 | 1 | – |
| Experiment B | 16 | 35 | 0.45 | 0.25–4 | – |
| Experiment C | 16 | – | – | 0.25–4 | – |
| Experiment D | 16 | 35 | – and 0.45 | 0.25–4 | 0.25–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizki, Z.; Janssen, A.E.M.; van der Padt, A.; Boom, R.M. Separation of Fructose and Glucose via Nanofiltration in Presence of Fructooligosaccharides. Membranes 2020, 10, 298. https://doi.org/10.3390/membranes10100298
Rizki Z, Janssen AEM, van der Padt A, Boom RM. Separation of Fructose and Glucose via Nanofiltration in Presence of Fructooligosaccharides. Membranes. 2020; 10(10):298. https://doi.org/10.3390/membranes10100298
Chicago/Turabian StyleRizki, Zulhaj, Anja E. M. Janssen, Albert van der Padt, and Remko M. Boom. 2020. "Separation of Fructose and Glucose via Nanofiltration in Presence of Fructooligosaccharides" Membranes 10, no. 10: 298. https://doi.org/10.3390/membranes10100298