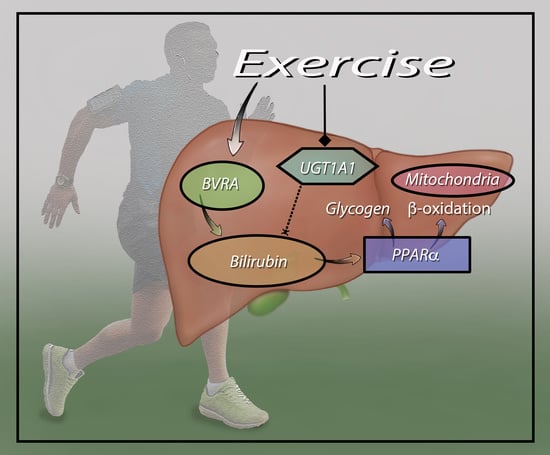
Rats Genetically Selected for High Aerobic Exercise Capacity Have Elevated Plasma Bilirubin by Upregulation of Hepatic Biliverdin Reductase-A (BVRA) and Suppression of UGT1A1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Liver Histology
2.3. Quantification of Small Molecules in Plasma
2.4. Quantitative Real-Time PCR Analysis
2.5. Bilirubin Measurements
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hinds, T.D., Jr.; Stec, D.E. Bilirubin Safeguards Cardiorenal and Metabolic Diseases: A protective role in health. Curr. Hypertens. Rep. 2019, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Stec, D.E. Bilirubin, a cardiometabolic signaling molecule. Hypertension 2018, 72, 788–795. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Lindroos, A.-K.; Sjöström, C.D.; Olsson, R.; Lissner, L.; Sjöström, L. Are elevated aminotransferases and decreased bilirubin additional characteristics of the metabolic syndrome? Obes. Res. 1997, 5, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yun, K.; Choi, H. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Guzek, M.; Jakubowski, Z.; Bandosz, P.; Wyrzykowski, B.; Smoczyński, M.; Jabloiska, A.; Zdrojewski, T. Inverse association of serum bilirubin with metabolic syndrome and insulin resistance in Polish population. Prz. Epidemiol. 2012, 66, 495–501. [Google Scholar]
- O’Brien, L.; Hosick, P.A.; John, K.; Stec, D.E.; Hinds, T.D. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015, 26, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Adeosun, S.O.; Moore, K.H.; Lang, D.M.; Nwaneri, A.C.; Hinds, T.D.; Stec, D.E. A novel fluorescence-based assay for the measurement of biliverdin reductase activity. React. Oxyg. Species 2018, 5, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Cimini, F.A.; Arena, A.; Barchetta, I.; Tramutola, A.; Ceccarelli, V.; Lanzillotta, C.; Fontana, M.; Bertoccini, L.; Leonetti, F.; Capoccia, D.; et al. Reduced biliverdin reductase—A levels are associated with early alterations of insulin signaling in obesity. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1490–1501. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Earnest, C.P.; Blair, S.N.; Church, T.S. Effect of different doses of aerobic exercise training on total bilirubin levels. Med. Sci. Sports Exerc. 2012, 44, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Witek, K.; Ścisłowska, J.; Turowski, D.; Lerczak, K.; Lewandowska-Pachecka, S.; Pokrywka, A. Total bilirubin in athletes, determination of reference range. Boil. Sport 2016, 34, 45–48. [Google Scholar] [CrossRef]
- Barrett, P.V. Hyperbilirubinemia of fasting. JAMA 1971, 217, 1349–1353. [Google Scholar] [CrossRef]
- Barrett, P.V. The effect of diet and fasting on the serum bilirubin concentration in the rat. Gastroenterology 1971, 60, 572–576. [Google Scholar] [CrossRef]
- Meyer, B.; Scholtz, H.; Schall, R.; Muller, F.; Hundt, H.; Maree, J. The effect of fasting on total serum bilirubin concentrations. Br. J. Clin. Pharmacol. 1995, 39, 169–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lin, Y.; Zhou, Z.; Gao, L.; Yang, Z.; Li, F.; Wu, B. Circadian clock gene Bmal1 regulates bilirubin detoxification: A potential mechanism of feedback control of hyperbilirubinemia. Theranostics 2019, 9, 5122–5133. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Hamoud, A.-R.A.; Stec, D.E.; Hinds, T.D. Biliverdin reductase and bilirubin in hepatic disease. Am. J. Physiol. Liver Physiol. 2018, 314, G668–G676. [Google Scholar] [CrossRef]
- Hamoud, A.-R.; Weaver, L.; Stec, D.E.; Hinds, T.D. Bilirubin in the liver–gut signaling axis. Trends Endocrinol. Metab. 2018, 29, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, V.L.; Sindhwani, P.; Hinds, T.D. Glucuronidation and UGT isozymes in bladder: New targets for the treatment of uroepithelial carcinomas? Oncotarget 2016, 8, 3640–3648. [Google Scholar] [CrossRef] [Green Version]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D., Jr. Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. Bilirubin binding to PPARalpha inhibits lipid accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.M.; Blomquist, T.M.; Miruzzi, S.A.; McCullumsmith, R.; Stec, D.E.; Hinds, T.D.; Hinds, T.D. RNA sequencing in human HepG2 hepatocytes reveals PPAR-α mediates transcriptome responsiveness of bilirubin. Physiol. Genom. 2019, 51, 234–240. [Google Scholar] [CrossRef]
- Gordon, D.M.; Neifer, K.L.; Hamoud, A.-R.A.; Hawk, C.F.; Nestor-Kalinoski, A.L.; Miruzzi, S.A.; Morran, M.P.; Adeosun, S.O.; Sarver, J.G.; Erhardt, P.W.; et al. Bilirubin remodels murine white adipose tissue by reshaping mitochondrial activity and the coregulator profile of peroxisome proliferator-activated receptor α. J. Boil. Chem. 2020, 295, 9804–9822. [Google Scholar] [CrossRef]
- Preidis, G.A.; Kim, K.H.; Moore, D.D. Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J. Clin. Investig. 2017, 127, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinds, T.D., Jr.; Hosick, P.A.; Chen, S.; Tukey, R.H.; Hankins, M.W.; Nestor-Kalinoski, A.; Stec, D.E. Mice with hyperbilirubinemia due to Gilbert’s syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARalpha. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E244–E252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinds, T.D., Jr.; Burns, K.A.; Hosick, P.A.; McBeth, L.; Nestor-Kalinoski, A.; Drummond, H.A.; AlAmodi, A.A.; Hankins, M.W.; Vanden Heuvel, J.P.; Stec, D.E. Biliverdin reductase a attenuates hepatic steatosis by inhibition of Glycogen Synthase Kinase (GSK) 3beta phosphorylation of Serine 73 of Peroxisome Proliferator-activated Receptor (PPAR) alpha. J. Biol. Chem. 2016, 291, 25179–25191. [Google Scholar] [CrossRef] [Green Version]
- Hinds, T.D.; Sodhi, K.; Meadows, C.; Fedorova, L.; Puri, N.; Kim, N.H.; Peterson, S.J.; Shapiro, J.; Abraham, N.G.; Kappas, A. Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21. Obesity 2013, 22, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Koch, L.G.; Britton, S.L. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genom. 2001, 5, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Wisløff, U.; Najjar, S.M.; Ellingsen, Ø.; Haram, P.M.; Swoap, S.; Al-Share, Q.; Fernström, M.; Rezaei, K.; Lee, S.J.; Koch, L.G.; et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 2005, 307, 418–420. [Google Scholar] [CrossRef] [Green Version]
- Thyfault, J.P.; Rector, R.S.; Uptergrove, G.M.; Borengasser, S.J.; Morris, E.M.; Wei, Y.; Laye, M.; Burant, C.F.; Qi, N.R.; Ridenhour, S.E.; et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J. Physiol. 2009, 587, 1805–1816. [Google Scholar] [CrossRef]
- Koch, L.G.; Kemi, O.J.; Qi, N.; Leng, S.X.; Bijma, P.; Gilligan, L.J.; Wilkinson, J.E.; Wisløff, H.; Høydal, M.A.; Rolim, N.; et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ. Res. 2011, 109, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Stec, D.E.; Gordon, D.M.; Hipp, J.A.; Hong, S.; Mitchell, Z.L.; Franco, N.R.; Robison, J.W.; Anderson, C.D.; Stec, D.F.; Hinds, T.D., Jr. Loss of hepatic PPARalpha promotes inflammation and serum hyperlipidemia in diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R733–R745. [Google Scholar] [CrossRef]
- Petersen, M.; Dyrby, M.; Toubro, S.; Engelsen, S.B.; Nørgaard, L.; Pedersen, H.T.; Dyerberg, J. Quantification of lipoprotein subclasses by proton nuclear magnetic resonance–based partial least-squares regression models. Clin. Chem. 2005, 51, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, J.S.; Stechschulte, L.A.; Stec, D.E.; Nestor-Kalinoski, A.; Coleman, S.; Hinds, T.D., Jr. Glucocorticoid receptor beta induces hepatic steatosis by augmenting inflammation and inhibition of the Peroxisome Proliferator-activated Receptor (PPAR) alpha. J. Biol. Chem. 2016, 291, 25776–25788. [Google Scholar] [CrossRef] [Green Version]
- Overmyer, K.A.; Evans, C.; Qi, N.R.; Minogue, C.E.; Carson, J.J.; Chermside-Scabbo, C.J.; Koch, L.G.; Britton, S.L.; Pagliarini, D.J.; Coon, J.J.; et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 2015, 21, 468–478. [Google Scholar] [CrossRef] [Green Version]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Yamamoto, T.; Ohkuwa, T.; Saito, M.; Miyamura, M. Peak blood lactate after short periods of maximal treadmill running. Graefe’s Arch. Clin. Exp. Ophthalmol. 1982, 48, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Noland, R.C.; Thyfault, J.P.; Henes, S.T.; Whitfield, B.R.; Woodlief, T.L.; Evans, J.R.; Lust, J.A.; Britton, S.L.; Koch, L.G.; Dudek, R.W.; et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am. J. Physiol. Metab. 2007, 293, E31–E41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandard, S.; Stienstra, R.; Escher, P.; Tan, N.S.; Kim, I.; Gonzalez, F.J.; Wahli, W.; Desvergne, B.; Muller, M.; Kersten, S. Glycogen synthase 2 is a novel target gene of peroxisome proliferator-activated receptors. Cell. Mol. Life Sci. 2007, 64, 1145–1157. [Google Scholar] [CrossRef] [Green Version]
- Stec, D.E.; Gordon, D.M.; Nestor-Kalinoski, A.L.; Donald, M.C.; Mitchell, Z.L.; Creeden, J.F.; Hinds, T.D. Biliverdin Reductase A (BVRA) knockout in adipocytes induces hypertrophy and reduces mitochondria in white fat of obese mice. Biomolecules 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Rego, A.; Cooper, K.F.; Snider, J.; Hannun, Y.A.; Costa, V.; Côrte-Real, M.; Chaves, S.R. Acetic acid induces Sch9p-dependent translocation of Isc1p from the endoplasmic reticulum into mitochondria. Biochim. Biophys. Acta Mol. Cell Boil. Lipids 2018, 1863, 576–583. [Google Scholar] [CrossRef]
- Pan, J.H.; Kim, J.-H.; Kim, H.M.; Lee, E.S.; Shin, N.-H.; Kim, S.; Shin, M.; Kim, S.H.; Park, J.-W.; Kim, Y.J. Acetic acid enhances endurance capacity of exercise-trained mice by increasing skeletal muscle oxidative properties. Biosci. Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, K.; He, J. Epidemiology of the metabolic syndrome. Am. J. Med Sci. 2005, 330, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Lawlor, M.W.; Mazariegos, G.V.; McKiernan, P.; Squires, J.E.; Strauss, K.A.; Gupta, D.; James, E.; Prasad, S. Disease burden of Crigler–Najjar syndrome: Systematic review and future perspectives. J. Gastroenterol. Hepatol. 2019, 35, 530–543. [Google Scholar] [CrossRef]
- Sundararaghavan, V.L.; Binepal, S.; Stec, D.E.; Sindhwani, P.; Hinds, T.D. Bilirubin, a new therapeutic for kidney transplant? Transplant. Rev. 2018, 32, 234–240. [Google Scholar] [CrossRef]
- Fevery, J.; Muraca, M.; Mesa, V.; Van Steenbergen, W.; Blanckaert, N. Plasma bilirubin pigments in health and disease. Mol. Asp. Med. 1987, 9, 391–404. [Google Scholar] [CrossRef]
- Fevery, J.; Vanstapel, F.; Blanckaert, N. Bile pigment metabolism. Baillière’s Clin. Gastroenterol. 1989, 3, 283–312. [Google Scholar] [CrossRef]
- Vítek, L.; Jirsa, M.; Brodanova, M.; Kaláb, M.; Marecek, Z.; Danzig, V.; Novotný, L.; Kotal, P. Gilbert syndrome and ischemic heart disease: A protective effect of elevated bilirubin levels. Atherosclerosis 2002, 160, 449–456. [Google Scholar] [CrossRef]
- Lin, J.-P.; O’Donnell, C.J.; Schwaiger, J.P.; Cupples, L.A.; Lingenhel, A.; Hunt, S.C.; Yang, S.; Kronenberg, F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the framingham heart study. Circulation 2006, 114, 1476–1481. [Google Scholar] [CrossRef] [Green Version]
- Jang, B.K. Elevated serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012, 18, 357–359. [Google Scholar] [CrossRef]
- Kwak, M.-S.; Kim, D.; Chung, G.E.; Kang, S.J.; Park, M.J.; Kim, Y.J.; Yoon, J.-H.; Lee, H.-S. Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012, 18, 383–390. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Xu, M.; Bi, Y.; Li, X.; Chen, Y.; Ning, G.; Wang, W. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J. Diabetes 2011, 3, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, P.; Gorrepati, V.S.; Peters, I.; Nookala, V.; Murphy, M.E.; Srouji, N.; Fischman, D. High total bilirubin as a protective factor for diabetes mellitus: An analysis of NHANES data from 1999–2006. J. Clin. Med. Res. 2010, 2, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.-M.; Kam, J.-H.; Kim, M.-Y.; Kim, M.Y.; Chung, C.H.; Kim, J.-K.; Linton, J.A.; Eom, A.; Koh, S.-B.; Kang, H.-T. Inverse association between total bilirubin and metabolic syndrome in rural Korean women. J. Women’s Health 2011, 20, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Yun, J.E.; Lee, H.; Kimm, H.; Jee, S.H. Total, direct, and indirect serum bilirubin concentrations and metabolic syndrome among the Korean population. Endocrine 2010, 39, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Na, K.Y.; Chae, D.-W.; Kim, Y.S.; Kim, S.; Chin, H.J. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. Tohoku, J. Exp. Med. 2010, 221, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflueger, A.; Croatt, A.J.; Peterson, T.E.; Smith, L.A.; D’Uscio, L.V.; Katusic, Z.S.; Nath, K. The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am. J. Physiol. Physiol. 2005, 288, F552–F558. [Google Scholar] [CrossRef]
- Vera, T.; Granger, J.P.; Stec, D.E. Inhibition of bilirubin metabolism induces moderate hyperbilirubinemia and attenuates ANG II-dependent hypertension in mice. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R738–R743. [Google Scholar] [CrossRef] [Green Version]
- Fallon, K.E.; Sivyer, G.; Sivyer, K.; Dare, A. The biochemistry of runners in a 1600 km ultramarathon. Br. J. Sports Med. 1999, 33, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Han, C.H.; Wang, Q.E.; Wang, S.; Shen, H.Q. Changes of the certain functions of mitochondria induced by acute exercise and the protective effect of bilirubin. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2001, 17, 72–75. [Google Scholar]
- Rivas, D.A.; Lessard, S.J.; Saito, M.; Friedhuber, A.M.; Koch, L.G.; Britton, S.L.; Yaspelkis, B.B.; Hawley, J.A. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R835–R843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, D.M.; Adeosun, S.O.; Ngwudike, S.I.; Anderson, C.D.; Hall, J.E.; Hinds, T.D.; Stec, D.E. CRISPR Cas9-mediated deletion of biliverdin reductase A (BVRA) in mouse liver cells induces oxidative stress and lipid accumulation. Arch. Biochem. Biophys. 2019, 672, 108072. [Google Scholar] [CrossRef] [PubMed]
- Kannisto, K.; Chibalin, A.V.; Glinghammar, B.; Zierath, J.R.; Hamsten, A.; Ehrenborg, E. Differential expression of peroxisomal proliferator activated receptors alpha and delta in skeletal muscle in response to changes in diet and exercise. Int. J. Mol. Med. 2006, 17, 45–52. [Google Scholar] [PubMed]
- Burri, L.; Thoresen, G.H.; Berge, R.K. The role of PPARα activation in liver and muscle. PPAR Res. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [Green Version]
- John, K.; Marino, J.S.; Sanchez, E.R.; Hinds, T.D. The glucocorticoid receptor: Cause of or cure for obesity? Am. J. Physiol. Metab. 2016, 310, E249–E257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraceni, P.; Viola, A.; Piscitelli, F.; Giannone, F.; Berzigotti, A.; Cescon, M.; Domenicali, M.; Petrosino, S.; Giampalma, E.; Riili, A.; et al. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2009, 30, 816–825. [Google Scholar] [CrossRef]
- Bigo, C.; Kaeding, J.; El Husseini, D.; Rudkowska, I.; Verreault, M.; Vohl, M.C.; Barbier, O. PPARalpha: A master regulator of bilirubin homeostasis. PPAR Res. 2014, 2014, 747014. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Adeosun, S.O.; Alamodi, A.A.; Stec, D.E. Does bilirubin prevent hepatic steatosis through activation of the PPARalpha nuclear receptor? Med. Hypotheses 2016, 95, 54–57. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinds, T.D., Jr.; Creeden, J.F.; Gordon, D.M.; Spegele, A.C.; Britton, S.L.; Koch, L.G.; Stec, D.E. Rats Genetically Selected for High Aerobic Exercise Capacity Have Elevated Plasma Bilirubin by Upregulation of Hepatic Biliverdin Reductase-A (BVRA) and Suppression of UGT1A1. Antioxidants 2020, 9, 889. https://doi.org/10.3390/antiox9090889
Hinds TD Jr., Creeden JF, Gordon DM, Spegele AC, Britton SL, Koch LG, Stec DE. Rats Genetically Selected for High Aerobic Exercise Capacity Have Elevated Plasma Bilirubin by Upregulation of Hepatic Biliverdin Reductase-A (BVRA) and Suppression of UGT1A1. Antioxidants. 2020; 9(9):889. https://doi.org/10.3390/antiox9090889
Chicago/Turabian StyleHinds, Terry D., Jr., Justin F. Creeden, Darren M. Gordon, Adam C. Spegele, Steven L. Britton, Lauren G. Koch, and David E. Stec. 2020. "Rats Genetically Selected for High Aerobic Exercise Capacity Have Elevated Plasma Bilirubin by Upregulation of Hepatic Biliverdin Reductase-A (BVRA) and Suppression of UGT1A1" Antioxidants 9, no. 9: 889. https://doi.org/10.3390/antiox9090889