Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Polyphenolic Extracts from Mamaia Grape Pomace
2.3. Synthesis of Pristine and Functionalized MCM-41 Supports
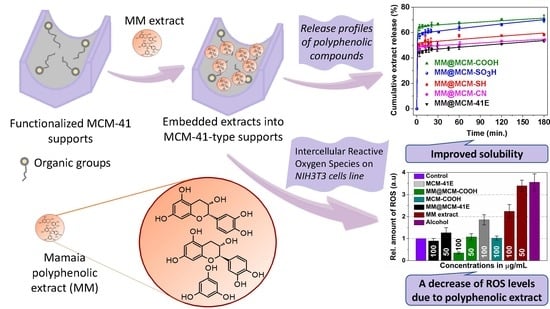
2.4. Loading Polyphenolic Extracts into Mesoporous Silica-Type Nanocarriers
2.5. Materials Characterization
2.6. Determination of Polyphenols Release Profiles
2.7. Evaluation of Cellular Viability and Oxidative Stress Level
3. Results
3.1. Polyphenolic Extracts Characterization
3.2. Characterization of Pristine and Functionalized Silica-Type Carriers
3.3. Characterization of Extract-Loaded Materials
3.4. Radical Scavenger Activity of Extract-Loaded Materials
3.5. Polyphenols Delivery Profiles from Pristine and Functionalized MCM-41 Supports
3.6. Evaluation of Cellular Viability and Oxidative Stress Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall material obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds -recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantili, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46–67. [Google Scholar] [CrossRef]
- Beres, C.; Simas-Tosin, F.F.; Cabezudo, I.; Freitas, S.P.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M. Antioxidant dietary fibre recovery from Brazilian Pinot noir grape pomace. Food Chem. 2016, 201, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beres, C.; Freitas, S.P.; de Oliveira Godoy, R.L.; de Oliveira, D.C.R.; Deliza, R.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M.C. Antioxidant dietary fibre from grape pomace flour or extract: Does it make any difference on the nutritional and functional value? J. Funct. Foods 2019, 56, 276–285. [Google Scholar] [CrossRef]
- Martinez, G.A.; Rbecchi, S.; Decorti, D.; Domingos, J.M.B.; Natolino, A.; Del Rio, D.; Bertin, L.; Da Porto, C.; Fava, F. Towards multi-purpose biorefinery platforms for the valorisation of red grape pomace: Production of polyphenols, volatile fatty acids, polyhydroxyalkanoates and biogas. Green Chem. 2016, 18, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape byproducts in monogastric nutrition. A review. Anim. Feed Sci. Tech. 2016, 211, 1–7. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [Green Version]
- Pinter, I.F.; Fernández, A.S.; Martínez, L.E.; Riera, N.; Fernández, M.; Aguado, G.D.; Uliarte, E.M. Exhausted grape marc and organic residues composting with polyethylene cover: Process and quality evaluation as plant substrate. J. Environ. Manag. 2019, 246, 695–705. [Google Scholar] [CrossRef]
- Martínez Salgado, M.M.; Ortega Blu, R.; Janssens, M.; Fincheira, P. Grape pomace compost as a source of organic matter: Evolution of quality parameters to evaluate maturity and stability. J. Clean. Prod. 2019, 216, 56–63. [Google Scholar] [CrossRef]
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queirós, A.; Costa, M.S.S.M.; Trindade, H. Comparison of five agro-industrial waste-based composts as growing media lettuce: Effect on yield, phenolic compounds and vitamin C. Food Chem. 2016, 209, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Morales, M.P.; Mendívil, M.A.; Juárez, M.C.; Muñoz, L. Using of waste pomace from winery industry to improve thermal insulation of fired clay bricks. Eco-friendly way of building construction. Constr. Build. Mater. 2014, 71, 181–187. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, R.D.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Yu, C.S.; Chiang, J.H.; Lu, C.C.; Yang, S.T.; Yu, C.C.; Chang, S.J.; et al. Gallic acid induces G(0)/G(1) phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer Res. 2011, 31, 2821–2832. [Google Scholar]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and poptotic death of DU-145 human prostate carcinoma cells. Carcinogenes 2006, 27, 1445–1453. [Google Scholar] [CrossRef]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Onning, G.; Akesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef]
- Dahiya, R.; Mohammad, T.; Roy, S.; Anwar, S.; Gupta, P.; Haque, A.; Khan, P.; Kazim, S.N.; Islam, A.; Ahmad, F.; et al. Investigation of inhibitory potential of quercetin to the pyruvate dehydrogenase kinase 3: Towards implications in anticancer therapy. Int. J. Biol. Macromol. 2019, 136, 1076–1085. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In Vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Kamel, K.M.; El-Raouf, A.; Ola, M.; Metwally, S.A.; El-Latif, A.; Hekma, A.; Elsayed, M.E. Hesperidin and rutin, antioxidant citrus flavonoids, attenuate cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxic. 2014, 28, 312–319. [Google Scholar] [CrossRef]
- Kane, C.J.M.; Menna, J.H.; Sung, C.-C.; Yeh, Y.-C. Methyl Gallate, Methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 1988, 8, 96–102. [Google Scholar]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Jang, H.J.; Kim, J.H.; Sohn, D.H.; Shin, D.W.; Park, H.; et al. In Vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J. Microbiol. Biotechnol. 2008, 18, 1848–1852. [Google Scholar]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef]
- Dai, Q.; Borenstein, A.R.; Wu, Y. Fruit and vegetable juices and Alzheimer’s disease: The Kame project. Am. J. Clin. Nutr. 2006, 119, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, O.; Mandel, S.; Amit, T.; Moussa, B.; Youdim, H. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Borai, H.I.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef] [Green Version]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Tech. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Fontana, R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Gaber Ahmed, G.H.; González, A.F.; Díaz García, M.E. Nano-encapsulation of grape and apple pomace phenolic extract in chitosan and soy protein via nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Tolun, A.; Artik, N.; Altintas, Z. Effect of different microencapsulating materials and relative humidities on storage stability of microencapsulated grape pomace extract. Food Chem. 2020, 302, 125347. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of Vitis vinifera (Grape)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2014, 33, 48–83. [Google Scholar] [CrossRef]
- Maluf, F.D.; Gonçalves, M.M.; D’Angelo, R.W.O.; Girassol, A.B.; Tulio, A.P.; Pupo, Y.M.; Farago, P.V. Cytoprotection of antioxidant biocompounds from grape pomace: Further exfoliant phytoactive ingredients for cosmetic products. Cosmetics 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The synergistic behavior of antioxidant phenolic compounds obtained from winemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Côté, M.-F.; Gaudreault, R.C.; Fortin, M.-A.; Kleitz, F. Size-controlled functionalized mesoporous silica nanoparticles for tunable drug release and enhanced anti-tumoral activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- Choi, S.R.; Jang, D.-J.; Kim, S.; An, S.; Lee, J.; Oh, E.; Kim, J. Polymer-coated spherical mesoporous silica for pH-controlled delivery of insulin. J. Mater. Chem. B 2014, 2, 616–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, M.; Ying, H.; Su, D.; Zhang, H.; Lu, G.; Chen, J. Extracellular matrix component shelled nanoparticles as dual enzyme-responsive drug delivery vehicles for cancer therapy. ACS Biomater. Sci. Eng. 2018, 4, 2404–2411. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Lincu, D.; Deaconu, M.; Mitran, R.-A.; Berger, D.; Matei, C. Enhanced stability of polyphenolic extracts from grape pomace achieved by embedding into mesoporous silica-type matrices. UPB Sci. Bull. B. accepted.
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Deaconu, M.; Nicu, I.; Vasile, E.; Mitran, R.-A.; Matei, C.; Berger, D. Heteroatom modified MCM-41-silica carriers for Lomefloxacin delivery systems. Micropor. Mesopor. Mater. 2019, 275, 222–241. [Google Scholar] [CrossRef]
- Deaconu, M.; Nicu, I.; Tincu, R.; Brezoiu, A.M.; Mitran, R.-A.; Vasile, E.; Matei, C.; Berger, D. Tailored doxycycline delivery from MCM-41-type silica carriers. Chem. Pap. 2018, 72, 1869–1880. [Google Scholar] [CrossRef]
- Deaconu, M.; Brezoiu, A.M.; Mitran, R.-A.; Nicu, I.; Manolescu, B.; Matei, C.; Berger, D. Exploiting the zwitterionic properties of lomefloxacin to tailor its delivery from functionalized MCM-41 silica. Micropor. Mesopor. Mater. 2020, 305, 112303. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Kong, H.; Zeng, Y.; Liu, G. Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment. Sci. Technol. Adv. Mat. 2018, 19, 771–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balea, S.S.; Parvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic compounds, antioxidant, and cardioprotective effects of pomace extracts from Fetească neagră cultivar. Oxid. Med. Cell. Longev. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Hornacek, M.; Hudec, P.; Smieskova, A. Synthesis and characterization of mesoporous molecular sieves. Chem. Pap. 2009, 63, 689–697. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; De Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crop. Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crop. Prod. 2018, 111, 379–390. [Google Scholar]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- García-Becerra, L.; Mitjans, M.; Rivas-Morales, C.; Verde-Star, J.; Oranday-Cárdenas, A.; María, P.V. Antioxidant comparative effects of two grape pomace Mexican extracts from vineyards on erythrocytes. Food Chem. 2016, 194, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manconi, M.; Marongiu, F.; Manca, M.L.; Caddeo, C.; Sarais, G.; Cencetti, C.; Pucci, L.; Longo, V.; Bacchetta, G.; Fadda, A.M. Nanoincorporation of bioactive compounds from red grape pomaces: In vitro and ex vivo evaluation of antioxidant activity. Int. J. Pharm 2017, 523, 159–166. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling Drug-Carrier Interaction in the Drug Release from Nanocarriers. J. Drug Deliv. 2011, 2011, 370308. [Google Scholar] [CrossRef] [PubMed]
- Mitran, R.-A.; Matei, C.; Berger, D. Correlation of mesoporous silica structural and morphological features with theoretical three-parameter model for drug release kinetics. J. Phys. Chem. C 2016, 120, 29202–29209. [Google Scholar] [CrossRef]
- Hao, N.; Li, L.; Tang, F. Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems. Int. Mater. Rev. 2017, 62, 57–77. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Gao, Y.; Shi, J.; Li, Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small 2009, 5, 2722–2729. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Hanuka Katz, I.; Eran Nagar, E.; Okun, Z.; Shpigelman, A. The Link between polyphenol structure, antioxidant capacity and shelf-life stability in the presence of fructose and ascorbic acid. Molecules 2020, 25, 225. [Google Scholar] [CrossRef] [Green Version]











| Extract | Extract (% wt) | TPC (mg GAE/ g) | TRS (mg AAE/ g) | TFC (mg QE/ g) | TAC (mg CGE/g) | IC50% (mg/ mL) | RSA DPPH (mg TE/ g) | RSA ABTS (mg TE/ g) |
|---|---|---|---|---|---|---|---|---|
| MM FI | 9.0 | 162.66 ± 1.59 | 16.86 ± 0.51 | 13.24 ± 1.37 | 7.51 ± 0.68 | 1.12 | 242.77 ± 5.93 | 208.02 ± 6.47 |
| MM FII | 4.4 | 167.74 ± 3.26 | 16.97 ± 1.33 | 9.31 ± 0.28 | 7.55 ± 0.44 | 1.02 | 265.74 ± 11.90 | 212.34 ± 5.03 |
| MM FIII | 2.7 | 187.89 ± 3.64 | 16.84 ± 0.42 | 8.17 ± 0.00 | 8.20 ± 0.13 | 0.97 | 280.19± 15.52 | 254.31 ± 9.06 |
| MM (Conv) | 15.5 | 196.32 ± 1.85 | 15.59± 0.41 | 10.31 ± 0.86 | 12.85 ± 0.23 | 1.10 | 246.58 ± 6.04 | 334.34 ± 14.45 |
| MM (MW) | 14.9 | 203.98 ± 1.15 | 7.30 ± 0.31 | 13.27 ± 0.04 | 13.03 ± 0.70 | 0.94 | 289.96 ± 10.47 | 313.33 ± 8.46 |
| Standard Substances | Concentration in Extract (mg/g) | |||||
|---|---|---|---|---|---|---|
| MM- Fr I | MM- Fr II | MM- Fr III | MM (Conv) | MM (MW) | MM (Conv)r | |
| gallic acid | 2.130 ± 0.003 | 1.821 ± 0.000 | 1.561 ± 0.001 | 2.118 ± 0.002 | 2.090 ± 0.000 | 2.420 ± 0.011 |
| protocatechuic acid | 0.532 ± 0.000 | 0.483 ± 0.001 | 0.464 ± 0.002 | 0.613 ± 0.002 | 0.577 ± 0.002 | 0.640 ± 0.004 |
| caftaric acid | 0.552 ± 0.000 | 0.857 ± 0.006 | 1.085 ± 0.006 | 0.794 ± 0.000 | 0.779 ± 0.004 | 0.318 ± 0.000 |
| catechin hydrate | 11.344 ± 0.016 | 9.964 ± 0.002 | 9.909 ± 0.032 | 10.050 ± 0.004 | 9.742 ± 0.000 | 8.571 ± 0.263 |
| vanillic acid | 1.238 ± 0.003 | 1.015 ± 0.001 | 0.818 ± 0.001 | 1.050 ± 0.003 | 1.063 ± 0.002 | 1.547 ± 0.006 |
| syringic acid | 0.654 ± 0.002 | 0.496 ± 0.002 | 0.416 ± 0.003 | 0.641 ± 0.000 | 0.642 ± 0.001 | 0.722 ± 0.012 |
| (−) epicatechin | 7.536 ± 0.017 | 6.672 ± 0.000 | 6.525 ± 0.002 | 6.784 ± 0.002 | 6.755 ± 0.012 | 5.560 ± 0.009 |
| p-coumaric acid | nd | nd | nd | 0.046 ± 0.001 | 0.043 ± 0.000 | 0.057 ± 0.002 |
| ellagic acid dihydrate | 0.237 ± 0.001 | 0.253 ± 0.003 | 0.212 ± 0.000 | 0.225 ± 0.000 | 0.227 ± 0.001 | 0.143 ± 0.000 |
| rutin hydrate | 0.739 ± 0.000 | 0.702 ± 0.001 | 0.555 ± 0.001 | 0.681 ± 0.000 | 0.738 ± 0.001 | 0.814 ± 0.000 |
| trans-resveratrol | nd | nd | nd | 0.053 ± 0.000 | 0.080 ± 0.000 | 0.075 ± 0.000 |
| quercetin | 0.805 ± 0.001 | 0.936 ± 0.001 | 1.166 ± 0.003 | 0.789 ± 0.001 | 0.758 ± 0.001 | 0.619 ± 0.009 |
| Support Type | Support | MM@support | ||||||
|---|---|---|---|---|---|---|---|---|
| nSiO2/nFG | dDFT (nm) | SBET (m2/g) | Vp (cm3/g) | Extract (% wt) | dDFT (nm) | SBET (m2/g) | Vp (cm3/g) | |
| MCM-41E | - | 3.93 | 781 | 0.69 | 42.0 | 3.66 | 97 | 0.11 |
| MCM-SH | 25 | 3.54 | 843 | 0.74 | 36.0 | 3.42 | 231 | 0.16 |
| MCM-SO3H | 50 | 3.66 | 798 | 0.59 | 38.5 | 3.54 | 141 | 0.10 |
| MCM-CN | 11 | 3.18 | 845 | 0.55 | 39.0 | - | 75 | 0.06 |
| MCM-COOH | 14 | 3.18 | 585 | 0.43 | 39.0 | - | 10 | 0.04 |
| Materials Containing Embedded Extract | Three-Parameter Fitting Equation | Maximum Amount of Extract Recovered (%) | ||||
|---|---|---|---|---|---|---|
| ΔG (1021J) | kd (min−1) | koff (103min−1) | kon (103min−1) | R2 | ||
| MM@MCM-41E | −0.76 | 1.940 | 0.899 | 1.073 | 0.9936 | 57.8 ± 0.6 |
| MM@MCM-SH | 0.31 | 2.256 | 0.985 | 0.917 | 0.9934 | 69.4 ± 0.4 |
| MM@MCM-SO3H | 1.57 | 2.256 | 1.756 | 1.218 | 0.9975 | 73.3 ± 1.9 |
| MM@MCM-CN | −0.07 | 2.256 | 0.721 | 0.732 | 0.9981 | 70.0 ± 1.4 |
| MM@MCM-COOH | 2.63 | 1.938 | 1.129 | 0.610 | 0.9993 | 73.5 ± 1.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Stoica Guzun, A.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. https://doi.org/10.3390/antiox9080696
Brezoiu A-M, Bajenaru L, Berger D, Mitran R-A, Deaconu M, Lincu D, Stoica Guzun A, Matei C, Moisescu MG, Negreanu-Pirjol T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants. 2020; 9(8):696. https://doi.org/10.3390/antiox9080696
Chicago/Turabian StyleBrezoiu, Ana-Maria, Laura Bajenaru, Daniela Berger, Raul-Augustin Mitran, Mihaela Deaconu, Daniel Lincu, Anicuta Stoica Guzun, Cristian Matei, Mihaela Georgeta Moisescu, and Ticuta Negreanu-Pirjol. 2020. "Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity" Antioxidants 9, no. 8: 696. https://doi.org/10.3390/antiox9080696