Changes in Oxidative Stress, Inflammation, and Muscle Damage Markers Following Diet and Beetroot Juice Supplementation in Elite Fencers
Abstract
:1. Introduction
2. Materials and Methods
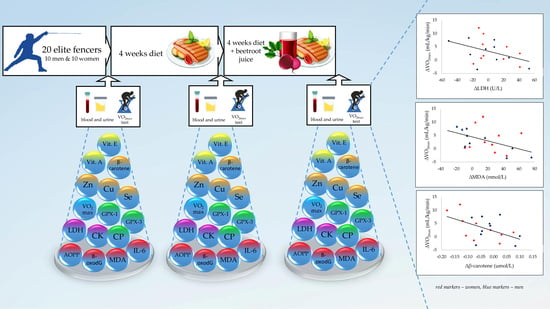
2.1. Participants and Study Design
- After all-night fasting, the blood and urine samples were collected. Then, body composition was measured.
- Afterward, a meal with a particular composition was consumed: Toasted bread (120 g), cold meats (sirloin, 60 g), banana (100 g), and a butter mix (24 g), as well as maltodextrin (26 g) or freeze-dried beetroot juice (26 g) dissolved in 150 ml of water. The nutritional value of this meal was as follows: Energy, 787 kcal; protein, 23 g; total fat, 27; total carbohydrate, 117 g. Before the physical test, the fencers consumed a meal with maltodextrin after the first and the second stages (B and ID), but after the third stage (ID&BEET), freeze-dried beetroot juice was added to the meal.
2.2. Anthropometric Parameters, Physical Activity Level, and Maximum Rate of Oxygen Uptake
2.3. Nutritional Recommendations for Athletes
2.4. Dietary Records and Analysis of Dietary Intake
2.5. Blood Sampling and Measurements of Antioxidants, Biomarkers of Oxidative Stress, Inflammation, and Skeletal Muscle Damage
2.6. Statistical Analysis
3. Results
3.1. Anthropometric Parameters, Physical Activity Level, and Maximum Rate of Oxygen Uptake
3.2. Energy and Nutrient Content in the Diet
3.3. Biomarkers of Oxidative Stress, Inflammation, and Skeletal Muscle Damage
3.4. Relationship Between the Analyzed Parameters
4. Discussion
5. Conclusions
Practical Application
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgiev, V.G.; Weber, J.; Kneschke, E.-M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant Activity and Phenolic Content of Betalain Extracts from Intact Plants and Hairy Root Cultures of the Red Beetroot Beta Vulgaris Cv. Detroit Dark Red. Plant. Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kujala, T.; Vienola, M.; Klika, K.; Loponen, J.; Pihlaja, K. Betalain and Phenolic Compositions of Four Beetroot (Beta Vulgaris) Cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. A Beetroot Juice Shot Is a Significant and Convenient Source of Bioaccessible Antioxidants. J. Funct. Foods 2011, 3, 329–334. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wettasinghe, M.; Bolling, B.W.; Ji, L.-L.; Parkin, K.L. Betalains, Phase II Enzyme-Inducing Components from Red Beetroot (Beta vulgaris L.) Extracts. Nutr. Cancer 2005, 53, 91–103. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In Vivo and in Vitro Antioxidant Effects of Beetroot Pomace Extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Zielińska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In Vitro Effects of Beetroot Juice and Chips on Oxidative Metabolism and Apoptosis in Neutrophils from Obese Individuals. Phytother. Res. 2009, 23, 49–55. [Google Scholar] [CrossRef]
- Pavlov, A.; Georgiev, V.; Ilieva, M. Betalain Biosynthesis by Red Beet (Beta Vulgaris L.) Hairy Root Culture. Process. Biochem. 2005, 40, 1531–1533. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, Excretion, and Distribution of Dietary Antioxidant Betalains in LDLs: Potential Health Effects of Betalains in Humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of Lipoxygenase and Cyclooxygenase by Natural Betalains and Semi-Synthetic Analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.M. Influence of Dietary Nitrate on the Physiological Determinants of Exercise Performance: A Critical Review. Appl. Physiol. Nutr. Metab. 2014, 39, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlström, M.; Larsen, F.J.; Weitzberg, E. Roles of Dietary Inorganic Nitrate in Cardiovascular Health and Disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the Antioxidant Effects of Nitric Oxide. Antioxid. Redox Signal. 2001, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.S.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric Oxide and Redox Mechanisms in the Immune Response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and Nitrite in Health and Disease. Aging Dis. 2018, 9, 938. [Google Scholar] [CrossRef] [Green Version]
- Hickner, R.C.; Fisher, J.S.; Ehsani, A.A.; Kohrt, W.M. Role of Nitric Oxide in Skeletal Muscle Blood Flow at Rest and during Dynamic Exercise in Humans. Am. J. Physiol. Heart Circ. Physiol. 1997, 273, H405–H410. [Google Scholar] [CrossRef]
- Gilligan, D.M.; Panza, J.A.; Kilcoyne, C.M.; Waclawiw, M.A.; Casino, P.R.; Quyyumi, A.A. Contribution of Endothelium-Derived Nitric Oxide to Exercise-Induced Vasodilation. Circulation 1994, 90, 2853–2858. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Wilkerson, D.P.; Tarr, J.; Benjamin, N. Dietary Nitrate Supplementation Reduces the O2 Cost of Low-Intensity Exercise and Enhances Tolerance to High-Intensity Exercise in Humans. J. Appl. Physiol. 2009, 107, 13. [Google Scholar] [CrossRef] [Green Version]
- Lansley, K.E.; Winyard, P.G.; Fulford, J.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Dietary Nitrate Supplementation Reduces the O 2 Cost of Walking and Running: A Placebo-Controlled Study. J. Appl. Physiol. 2011, 110, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute Dietary Nitrate Supplementation Improves Cycling Time Trial Performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.; Eliot, K.; Heuertz, R.M.; Weiss, E. Whole Beetroot Consumption Acutely Improves Running Performance. J. Acad. Nutr. Diet. 2012, 112, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The Effect of Nitrate Supplementation on Exercise Performance in Healthy Individuals: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, H.; Morton, L.; Braakhuis, A.J. Dietary Nitrate Supplementation Improves Rowing Performance in Well-Trained Rowers. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Breese, B.C.; McNarry, M.A.; Marwood, S.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Beetroot Juice Supplementation Speeds O2 Uptake Kinetics and Improves Exercise Tolerance during Severe-Intensity Exercise Initiated from an Elevated Metabolic Rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1441–R1450. [Google Scholar] [CrossRef] [Green Version]
- Cermak, N.M.; Gibala, M.J.; van Loon, L.J.C. Nitrate Supplementation’s Improvement of 10-Km Time-Trial Performance in Trained Cyclists. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 64–71. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.; Stinkens, R.; Lundberg, J.O.; Gibala, M.J.; van Loon, L.J.C. No Improvement in Endurance Performance after a Single Dose of Beetroot Juice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.M.; Nyberg, M.; Bangsbo, J. Influence of Nitrate Supplementation on VO2 Kinetics and Endurance of Elite Cyclists: Nitrate Supplementation in Elite Cyclists. Scand. J. Med. Sci. Sports 2013, 23, e21–e31. [Google Scholar] [CrossRef]
- Muggeridge, D.J.; Howe, C.C.F.; Spendiff, O.; Pedlar, C.; James, P.E.; Easton, C. A Single Dose of Beetroot Juice Enhances Cycling Performance in Simulated Altitude. Med. Sci. Sports Exerc. 2013, 46, 143–150. [Google Scholar] [CrossRef]
- Bescós, R.; Ferrer-Roca, V.; Galilea, P.A.; Roig, A.; Drobnic, F.; Sureda, A.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Sodium Nitrate Supplementation Does Not Enhance Performance of Endurance Athletes. Med. Sci. Sports Exerc. 2012, 44, 2400–2409. [Google Scholar] [CrossRef]
- Bescós’, R.; Rodríguez’, F.A.; Iglesias’, X.; Pons, A. Acute Administration of Inorganic Nitrate Reduces V02peak in Endurance Athletes. Med. Sci. Sports Exerc. 2011, 43, 1979–1986. [Google Scholar] [CrossRef] [Green Version]
- Trexler, E.T.; Keith, D.S.; Lucero, A.A.; Stoner, L.; Schwartz, T.A.; Persky, A.M.; Ryan, E.D.; Smith-Ryan, A.E. Effects of Citrulline Malate and Beetroot Juice Supplementation on Energy Metabolism and Blood Flow During Submaximal Resistance Exercise. J. Diet. Suppl. 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Keith, D.S.; Schwartz, T.A.; Ryan, E.D.; Stoner, L.; Persky, A.M.; Smith-Ryan, A.E. Effects of Citrulline Malate and Beetroot Juice Supplementation on Blood Flow, Energy Metabolism, and Performance During Maximum Effort Leg Extension Exercise. J. Strength Cond. Res. 2019, 33, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Vollaard, N.B.J.; Choueiri, T.; Wilson, M.T. Exercise, Free Radicals and Oxidative Stress. Biochem. Soc. Trans. 2002, 30, 6. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Mitochondria in Exercise-Induced Oxidative Stress. Neurosignals 2001, 10, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, E.; Northoff, H. Free Radicals, Exercise, Apoptosis, and Heat Shock Proteins. Exerc. Immunol. Rev. 2001, 7, 66–89. [Google Scholar]
- Gunther, M.R.; Sampath, V.; Caughey, W.S. Potential Roles of Myoglobin Autoxidation in Myocardial Ischemia-Reperfusion Injury. Free Radic. Biol. Med. 1999, 26, 1388–1395. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What Role Do They Play in Physical Activity and Health? Am. J. Clin. Nutr. 2000, 72, 637S–646S. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Packer, L. Antioxidant and Redox Regulation of Gene Transcription. FASEB J. 1996, 10, 709–720. [Google Scholar] [CrossRef]
- Reid, M.B. Invited Review: Redox Modulation of Skeletal Muscle Contraction: What We Know and What We Don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, D. Marcers of Excessive Exercise. Can. J. Appl. Physiol. 1999, 24, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Cazorla, G.; Poortmans, J.-R.; Déléris, G. Biochemical Aspects of Overtraining in Endurance Sports. Sports Med. 2002, 32, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An Introduction to Free Radical Biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Szweda, P.A.; Friguet, B.; Szweda, L.I. Serial Review: Oxidatively Modified Proteins in Aging and Disease. Free Radic. Biol. Med. 2002, 33, 29–36. [Google Scholar] [CrossRef]
- Wallace, S.S. Biological Consequences of Free Radical-Damaged DNA Bases. Free Radic. Biol. Med. 2002, 33, 1–14. [Google Scholar] [CrossRef]
- Radák, Z.; Pucsok, J.; Mecseki, S.; Csont, T.; Ferdinandy, P. Muscle Soreness-Induced Reduction in Force Generation Is Accompanied by Increased Nitric Oxide Content and DNA Damage in Human Skeletal Muscle. Free Radic. Biol. Med. 1999, 26, 1059–1063. [Google Scholar] [CrossRef]
- Petibois, C.; Cazorla, G.; Poortmans, J.-R.; Deleris, G. Biochemical Aspects of Overtraining in Endurance Sports. Sports Med. 2003, 33, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, L.; Mizera, O.; Mroz, A. An Untargeted Metabolomics Approach to Investigate the Metabolic Effect of Beetroot Juice Supplementation in Fencers—A Preliminary Study. Metabolites 2020, 10, 100. [Google Scholar] [CrossRef] [Green Version]
- Van Maanen, J.; van Geel, A.; Kleinjans, J. Modulation of Nitrate-Nitrite Conversion in the Oral Cavity. Cancer Detect. Prev. 1996, 20, 590–596. [Google Scholar]
- Kyle, U. Bioelectrical Impedance Analysis?Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An Update of Activity Codes and MET Intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, P.B.; Shing, C.M.; Peake, J.M.; Coombes, J.S.; Jenkins, D.G. Interval Training Program Optimization in Highly Trained Endurance Cyclists. Med. Sci. Sports Exerc. 2002, 34, 1801–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN Exercise & Sport Nutrition Review: Research & Recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Potgieter, S. Sport Nutrition: A Review of the Latest Guidelines for Exercise and Sport Nutrition from the American College of Sport Nutrition, the International Olympic Committee and the International Society for Sports Nutrition. South. Afr. J. Clin. Nutr. 2013, 26, 6–16. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Wierzejska, R.; Wojtasik, A.; Charzewska, J.; Mojska, H.; Szponar, L.; Sajór, I.; Kłosiewicz-Latoszek, L.; et al. Normy żywienia dla populacji Polski; Instytut Żywności i Żywienia: Warsaw, Poland, 2017. [Google Scholar]
- Grzelińska, Z.; Gromadzińska, J.; Swiercz, R.; Wąsowicz, W. Plasma Concentrations of Vitamin E, Vitamin A and Beta-Carotene in Healthy Men. Pol. J. Environ. Stud. 2007, 16, 209–213. [Google Scholar]
- Janasik, B.; Zawisza, A.; Malachowska, B.; Fendler, W.; Stanislawska, M.; Kuras, R.; Wasowicz, W. Relationship between Arsenic and Selenium in Workers Occupationally Exposed to Inorganic Arsenic. J. Trace Elem. Med. Biol. 2017, 42, 76–80. [Google Scholar] [CrossRef]
- Sunderman, F.W.; Nomoto, S. Measurement of Human Serum Ceruloplasmin by Its P-Phenylenediamine Oxidase Activity. Clin. Chem. 1970, 16, 903–910. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Goran, M.; Fields, D.; Hunter, G.; Herd, S.; Weinsier, R. Total Body Fat Does Not Inluence Maximalaerobic Capacity. Int. J. Obes. 2000, 24, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Więcek, M.; Szymura, J.; Szyguła, Z.; Wiecha, S.; Cempla, J. The Influence of Increased Body Fat or Lean Body Mass on Aerobic Performance. PLoS ONE 2014, 9, e95797. [Google Scholar] [CrossRef]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.L.; Muls, E. Exercise Capacity in Lean versus Obese Women. Scand. J. Med. Sci. Sports 2001, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Güzel, N.A.; Hazar, S.; Erbas, D. Effects of Different Resistance Exercise Protocols on Nitric Oxide, Lipid Peroxidation and Creatine Kinase Activity in Sedentary Males. J. Sports Sci. Med. 2007, 6, 417–422. [Google Scholar] [PubMed]
- Lovlin, R.; Cottle, W.; Pyke, I.; Kavanagh, M.; Belcastro, A.N. Are Indices of Free Radical Damage Related to Exercise Intensity. Eur. J. Appl. Physiol. 1987, 56, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Moflehi, D.; Kok, L.-Y.; Tengku-Kamalden, T.-F.; Amri, S. Effect of Single-Session Aerobic Exercise with Varying Intensities on Lipid Peroxidation and Muscle-Damage Markers in Sedentary Males. Glob. J. Health Sci. 2012, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Hasibuan, R.; Sinaga, F.A.; Sinaga, R.N. Effect of Beetroot Juice (Beta vulgaris L) During Training on Malondialdehyde Level in Athletes. Int. J. Sci. Res. 2018, 8, 4. [Google Scholar]
- Metin, G.; Gümüştaş, M.; Uslu, E.; Belce, A.; Kayserilioglu, A. Effect of Regular Training on Plasma Thiols, Malondialdehyde and Carnitine Concentrations in Young Soccer Players. Chin. J. Physiol. 2003, 46, 35–39. [Google Scholar]
- Shing, C.M.; Peake, J.M.; Ahern, S.M.; Strobel, N.A.; Wilson, G.; Jenkins, D.G.; Coombes, J.S. The Effect of Consecutive Days of Exercise on Markers of Oxidative Stress. Appl. Physiol. Nutr. Metab. 2007, 32, 677–685. [Google Scholar] [CrossRef]
- Foschini, D. Acute Hormonal and Immune Responses after a Bi-Set Strength Training. Fit. Perform. J. 2007, 6, 38–44. [Google Scholar] [CrossRef]
- Rodrigues, B.M.; Dantas, E.; de Salles, B.F.; Miranda, H.; Koch, A.J.; Willardson, J.M.; Simão, R. Creatine Kinase and Lactate Dehydrogenase Responses After Upper-Body Resistance Exercise With Different Rest Intervals. J. Strength Cond. Res. 2010, 24, 1657–1662. [Google Scholar] [CrossRef]
- Paschalis, V.; Giakas, G.; Baltzopoulos, V.; Jamurtas, A.Z.; Theoharis, V.; Kotzamanidis, C.; Koutedakis, Y. The Effects of Muscle Damage Following Eccentric Exercise on Gait Biomechanics. Gait Posture 2007, 25, 236–242. [Google Scholar] [CrossRef]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses After Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet. 2017, 58, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschalis, V.; Koutedakis, Y.; Jamurtas, A.Z.; Mougios, V.; Baltzopoulos, V. Equal Volumes of High and Low Intensity of Eccentric Exercise in Relation to Muscle Damage and Performance. J. Strength Cond. Res. 2005, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S.P.; Clarkson, P.M. Short-Term Immobilization after Eccentric Exercise. Part II: Creatine Kinase and Myoglobin: Med. Sci. Sports Exerc. 2003, 35, 762–768. [Google Scholar] [CrossRef]
- Gladden, L.B. The Basic Science of Exercise Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2222–2223. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate Metabolism: Historical Context, Prior Misinterpretations, and Current Understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Bohr, C.; Hasselbalch, K.; Krogh, A. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt1. Skand. Arch. Für Physiol. 1904, 16, 402–412. [Google Scholar] [CrossRef]
- Chatel, B.; Messonnier, L.A.; Bendahan, D. Do We Have to Consider Acidosis Induced by Exercise as Deleterious in Sickle Cell Disease? Exp. Physiol. 2018, 103, 1213–1220. [Google Scholar] [CrossRef]
- Palli, D.; Decarli, A.; Russo, A.; Cipriani, F.; Giacosa, A.; Amadori, D.; Salkeld, R.; Salvini, S.; Buiatti, E. Plasma Levels of Antioxidant Vitamins and Cholesterol in a Large Population Sample in Central-Northern Italy. Eur. J. Nutr. 1999, 38, 90–98. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Ferreira, A.L.A.; Epstein, S.; Paiva, S.A.; Castaneda-Sceppa, C.; Johnson, E.J. Site-Specific Concentrations of Carotenoids in Adipose Tissue: Relations with Dietary and Serum Carotenoid Concentrations in Healthy Adults. Am. J. Clin. Nutr. 2009, 90, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Faure, H.; Preziosi, P.; Roussel, A.-M.; Bertrais, S.; Galan, P.; Hercberg, S.; Favier, A. Factors Influencing Blood Concentration of Retinol, a-Tocopherol, Vitamin C, and b-Carotene in the French Participants of the SU.VI.MAX Trial. Eur. J. Clin. Nutr. 2006, 60, 706–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative Stress: Relationship with Exercise and Training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Ji, L. Oxidative Stress during Exercise: Implication of Antioxidant Nutrients. Free Radic. Biol. Med. 1995, 18, 1079–1086. [Google Scholar] [CrossRef]
- Inal, M.; Akyüz, F.; Turgut, A.; Mills Getsfrid, W. Effect of Aerobic and Anaerobic Metabolism on Free Radical Generation Swimmers. Med. Sci. Sports Exerc. 2001, 33, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Ji, L. Antioxidant Enzyme Response to Exercise and Aging. Med. Sci. Sports Exerc. 1993, 2, 225–231. [Google Scholar] [CrossRef]
- Powers, S.K.; Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G. Influence of Exercise and Fiber Type on Antioxidant Enzyme Activity in Rat Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hollander, J.; Leichtweis, S.; Griffiths, M.; Gore, M.; Ji, L.L. Adaptations of Glutathione Antioxidant System to Endurance Training Are Tissue and Muscle Fiber Specific. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 272, R363–R369. [Google Scholar] [CrossRef]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High Intensity Training-Induced Changes in Skeletal Muscle Antioxidant Enzyme Activity. Med. Sci. Sports Exerc. 1993, 10, 1135–1140. [Google Scholar] [CrossRef]
- Dehghan, G.; Shaghaghi, M.; Jafari, A.; Badalzadeh, R. Effect of Endurance Training and Cinnamon Supplementation on Post-Exercise Oxidative Responses in Rats. Mol. Biol. Res. Commun. 2014, 3, 269–281. [Google Scholar]
- Cases, N.; Sureda, A.; Maestre, I.; Tauler, P.; Aguiló, A.; Córdova, A.; Roche, E.; Tur, J.A.; Pons, A. Response of Antioxidant Defences to Oxidative Stress Induced by Prolonged Exercise: Antioxidant Enzyme Gene Expression in Lymphocytes. Eur. J. Appl. Physiol. 2006, 98, 263–269. [Google Scholar] [CrossRef]
- Elosua, R.; Molina, L.; Fito, M.; Arquer, A.; Sanchez-Quesada, J.L.; Covas, M.I.; Ordoñez-Llanos, J.; Marrugat, J. Response of Oxidative Stress Biomarkers to a 16-Week Aerobic Physical Activity Program, and to Acute Physical Activity, in Healthy Young Men and Women. Atherosclerosis 2003, 167, 327–334. [Google Scholar] [CrossRef]
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. The Effects of Beetroot Juice Supplementation on Indices of Muscle Damage Following Eccentric Exercise. Eur. J. Appl. Physiol. 2016, 116, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running: Cherry Juice Supplementation and Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jimenez, R.; Michałowski, T.; Wybraniec, S. Influence of Betalain-Rich Extract on Reduction of Discomfort Associated with Osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Twomey, P.J.; Viljoen, A.; House, I.M.; Reynolds, T.M.; Wierzbicki, A.S. Relationship between Serum Copper, Ceruloplasmin, and Non–Ceruloplasmin-Bound Copper in RoutineClinical Practice. Clin. Chem. 2005, 51, 1558–1559. [Google Scholar] [CrossRef]
- Linder, M.C. Ceruloplasmin and Other Copper Binding Components of Blood Plasma and Their Functions: An Update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef]
- Bielli, P.; Calabrese, L. Structure to Function Relationships in Ceruloplasmin: A “moonlighting” Protein. Cell. Mol. Life Sci. CMLS 2002, 59, 1413–1427. [Google Scholar] [CrossRef]
- Surina-Marysheva, E.F.; Krivokhizhina, L.V.; Kantyukov, S.A.; Sergienko, V.I.; Ermolaeva, E.N.; Smirnov, D.M. Effect of Ceruloplasmin on the Number and Resistance of Erythrocytes during Acute Physical Exercise. Bull. Exp. Biol. Med. 2009, 148, 188–190. [Google Scholar] [CrossRef]
- Liesen, H.; Dufaux, B.; Hollmann, W. Modifications of Serum Glycoproteins the Days Following a Prolonged Physical Exercise and the Influence of Physical Training. Eur. J. Appl. Physiol. 1977, 37, 243–254. [Google Scholar] [CrossRef]
- Resina, A.; Fedi, S.; Gatteschi, L.; Rubenni, M.; Giamberardino, M.; Trabassi, E.; Imreh, F. Comparison of Some Serum Copper Parameters in Trained Runners and Control Subjects. Int. J. Sports Med. 1990, 11, 58–60. [Google Scholar] [CrossRef]



| Variable | Stages of the Study | p b | pc | ||
|---|---|---|---|---|---|
| B | After ID | After ID&BEET | |||
| Women | |||||
| Weight (kg) | 63.6 ± 8.0 | 63.5 ± 8.3 | 63.6 ± 8.6 | 0.743 | 0.801 |
| FM% | 26.4 ± 4.3 | 25.91 ± 5.2 | 24.9 ± 4.8 | 0.303 | 0.059 |
| FFM% | 73.6 ± 4.3 | 74.09 ± 5.2 | 75.1 ± 4.8 | 0.303 | 0.059 |
| FM (kg) | 16.9 ± 4.0 | 16.5 ± 5.1 | 16.1 ± 4.8 | 0.339 | 0.214 |
| FFM (kg) | 46.7 ± 5.8 | 47.0 ± 4.9 | 47.5 ± 5.3 | 0.562 | 0.146 |
| VO2max(mL/kg/min) | 39.3 ± 4.8 | 39.4 ± 4.8 | 42.8 ± 4.7 | 0.668 | 0.011 |
| PA | 1.7 ± 0.2 | 1.8 ± 0.1 | 1.8 ± 0.1 | 0.325 | 0.544 |
| Men | |||||
| Weight (kg) | 82.5 ± 11.6 * | 82.4 ± 11.9 * | 82.7 ± 11.4 * | 0.781 | 0.529 |
| FM% | 17.7 ± 4.7 * | 16.9 ± 4.3 * | 16.6 ± 4.1 * | 0.123 | 0.584 |
| FFM% | 82.3 ± 4.7 * | 83.1 ± 4.3 * | 83.4 ± 4.1 * | 0.123 | 0.584 |
| FM (kg) | 15.0 ± 4.8 | 14.3 ± 4.5 | 14.0 ± 4.3 | 0.068 | 0.505 |
| FFM (kg) | 67.6 ± 8.2 * | 68.2 ± 8.5 * | 68.7 ± 8.4 * | 0.120 | 0.210 |
| VO2max(mL/kg/min) | 46.0 ± 7.8 * | 45.3 ± 9.3 | 48.5 ± 10.3 | 0.728 | 0.023 |
| PA | 1.9 ± 0.3 * | 1.9 ± 0.1 | 1.9 ± 0.1 | 0.088 | 0.659 |
| Variable | Stages of the Study | pb | pc | ||
|---|---|---|---|---|---|
| B | During ID | During ID&BEET | |||
| Absolute and relative energy and macronutrient intake | |||||
| Energy kcal/kg bw | 30.9 ± 7.4 | 32.6 ± 6.1 | 34.5 ± 6.1 | 0.120 | 0.084 |
| Carbohydrates (%E) | 45.6 ± 8.2 | 45.4 ± 4.2 | 46.6 ± 5.3 | 0.880 | 0.395 |
| Protein (%E) | 17.2 (11.3–31.2) | 18.0 (14.7–30.9) | 17.1 (14.1–26.2) | 0.269 | 0.023 |
| Fat (%E) | 32.9 (26.9–52.2) | 35.5 ± 3.3 | 35.4 ± 4.7 | 0.881 | 0.901 |
| SFA (%E) | 12.9 ± 4.5 | 12.7 ± 2.7 | 12.3 ± 2.4 | 0.776 | 0.608 |
| MUFA (%E) | 13.7 (9.0–27.5) | 14.8 ± 5.0 | 15.0 ± 3.0 | 0.794 | 0.841 |
| PUFA (%E) | 5.49 (2.30–11.19) | 5.22 (3.21–9.50) | 5.17 (3.74–9.32) | 0.391 | 0.601 |
| Nutrient intake in relation to the reference range | |||||
| Vit. E (%R) | 152.4 ± 50.3 | 148.7 ± 37.0 | 155.1 ± 35.6 | 0.746 | 0.573 |
| Vit. A (%R) | 148.8 ± 49.9 | 166.7±40.9 | 157.2 ± 41.4 | 0.367 | 0.913 |
| Vit. C (%R) | 148.4 ± 58.8 | 166.3 ± 72.2 | 162.8 ± 49.3 | 0.352 | 0.905 |
| Vit. B1 (%R) | 124.1 ± 39.3 | 124.4 ± 22.2 | 125.6 ± 24.1 | 0.780 | 0.900 |
| Vit. B2 (%R) | 168.8 ± 52.3 | 170.9 ± 34.5 | 168.8 ± 31.5 | 0.785 | 0.785 |
| Vit. B6 (%R) | 170.2 ± 58.7 | 184.4 ± 39.9 | 181.6 ± 40.8 | 0.262 | 0.827 |
| Vit. B12 (%R) | 182.6 (62.0–929.3) | 201.7 (111.0–662.7) | 177.1 (110.4–662.7) | 0.526 | 0.020 |
| Niacin (%R) | 137.8 ± 61.2 | 162.0 ± 36.5 | 155.5 ± 40.7 | 0.056 | 0.604 |
| Folate (%R) | 93.4 ± 30.9 | 91.0 ± 23.1 | 90.9 ± 18.4 | 0.700 | 0.986 |
| Zinc (%R) | 140.5 ± 40.0 | 143.3 ± 34.6 | 141.3 ± 31.8 | 0.638 | 0.730 |
| Iron (%R) | 112.9 ± 60.0 | 109.1 ± 44.3 | 107.3 ± 40.40 | 0.530 | 0.763 |
| Copper (%R) | 154.0 (77.7–348.2) | 161.4 (103.4–286.6) | 160.2 (131.9–253.7) | 1.000 | 0.496 |
| Calcium (%R) | 92.7 ± 39.0 | 98.9 ± 33.3 | 92.0 ± 21.6 | 0.785 | 0.949 |
| Magnesium (%R) | 106.8 ± 32.5 | 113.4 ± 25.3 | 112.7 ± 22.5 | 0.055 | 0.874 |
| Sodium (%R) | 249.5 ± 87.2 | 248.7 ± 69.3 | 234.6 ± 55.2 | 0.945 | 0.236 |
| Potassium (%R) | 98.6 ± 25.6 | 109.7 ± 21.1 | 110.2 ± 19.8 | 0.054 | 0.790 |
| Variable | Stages of the Study | pb | pc | ||
|---|---|---|---|---|---|
| B | After ID | After ID&BEET | |||
| Vit. E (mg/L) | 10.520 ± 2.331 | 11.335 ± 2.469 | 10.912 ± 3.069 | 0.067 | 0.334 |
| Vit. A (mg/L) | 0.625 ± 0.106 | 0.659 ± 0.111 | 0.633 ± 0.130 | 0.138 | 0.251 |
| β-carotene (µmol/L) | 0.302 ± 0.135 | 0.307 ± 0.127 | 0.273 ± 0.115 | 0.764 | 0.089 |
| Selenium (µg/L) | 63.1 ± 15.2 | 55.3 ± 8.5 | 64.2 ± 13.8 | 0.018 | 0.008 |
| Zinc (µg/L) | 790.9 ± 234.9 | 707.6 ± 142.5 | 819.1 ± 204.0 | 0.176 | 0.073 |
| Copper (µg/L) | 890.5 (626.4–1689.2) | 759.2 (599.8–2215.1) | 786.9 (529.2–1768.0) | 0.575 | 0.881 |
| CP (u/L) | 0.355 ± 0.161 | 0.426 ± 0.161 | 0.403 ± 0.217 | 0.115 | 0.608 |
| GPx-1 (u/g HGB) | 20.71 ± 4.54 | 22.99 ± 4.45 | 25.01 ± 3.78 | 0.000 | 0.012 |
| GPx-3 (u/mL) | 0.161 ± 0.024 | 0.171 ± 0.028 | 0.167 ± 0.023 | 0.006 | 0.233 |
| AOPP (µmol/L) | 94.02 ± 28.91 | 102.15 ± 34.70 | 89.35 ± 27.37 | 0.350 | 0.145 |
| MDA (nmol/L) | 80.52 ± 21.75 | 73.36 ± 16.36 | 90.86 ± 31.78 | 0.190 | 0.002 |
| 8-axodG (nmol/g cr) | 32.49 ± 18.47 | 33.27 ± 18.16 | 33.58 ± 19.02 | 0.841 | 0.937 |
| IL-6 (pg/mL) | 4.305 (0.100–59.960) | 3.215 (0.100–54.750) | 3.725 (0.100–36.510) | 0.438 | 0.679 |
| LDH (U/L) | 116.80 ± 33.38 | 128.25 ± 37.83 | 130.15 ± 33.68 | 0.048 | 0.737 |
| CK (U/L) | 75.00 (24.00–409.00) | 95.55 ± 51.66 | 109.00 (28.00–310.00) | 0.629 | 0.051 |
| Related Variables | Stage ID | Stage ID&BEET |
|---|---|---|
| PA versus VO2max (mL/kg/min) | r = 0.692, p = 0.001 | r = 0.632, p = 0.004 |
| FFM% versus VO2max (mL/kg/min) | r = 0.496, p = 0.026 | r = 0.471, p = 0.042 |
| FFM% versus LDH (U/L) | r = 0.434, p = 0.056 | r = 0.511, p = 0.021 |
| FFM% versus β-carotene (µmol/L) | r = −0.610, p = 0.004 | r = −0.555, p = 0.011 |
| LDH (U/L) versus β-carotene (µmol/L) | r = −0.468, p = 0.037 | r = −0.479, p = 0.033 |
| ∆LDH (U/L) versus ∆MDA (nmol/L) | r = 0.588, p = 0.006 | r = 0.465, p = 0.045 |
| ∆β-carotene (µmol/L) versus ∆VO2max (mL/kg/min) | r = 0.130, p = 0.584 | r = −0.500, p = 0.029 |
| ∆β-carotene (µmol/L) versus ∆AOPP (µmol/L) | r = 0.278, p = 0.235 | r = 0.623, p = 0.003 |
| ∆LDH (U/L) versus ∆VO2max (mL/kg/min) | r = 0.239, p = 0.311 | r = −0.518, p = 0.028 |
| ∆MDA (nmol/L) versus ∆VO2max (mL/kg/min) | r = 0.108, p = 0.651 | r = −0.472, p = 0.036 |
| LDH (U/L) versus CK (U/L) | r = 0.404, p = 0.078 | r = 0.620, p = 0.004 |
| ∆PA versus ∆GPx-3 (U/mL) | r = −0.171, p = 0.472 | r = 0.471, p = 0.036 |
| MDA (nmol/L) versus GPx-3 (u/mL) | r = −0.033, p = 0.891 | r = 0.447, p = 0.048 |
| ∆LDH (U/L) versus ∆GPx-3 (u/mL) | r = −0.011, p = 0.964 | r = 0.600, p = 0.007 |
| ∆LDH (U/L) versus ∆GPx-1 (u/g HGB) | r = 0.011, p = 0.963 | r = 0.467, p = 0.044 |
| ∆CP (u/L) versus ∆IL-6 (pg/mL) | r = −0.230, p = 0.360 | r = −0.486, p = 0.041 |
| GPx-3 (u/mL) versus Selenium (µg/L) | r = 0.456, p = 0.043 | r = 0.196, p = 0.408 |
| GPx-3 (u/mL) versus AOPP (µmol/L) | r = 0.528, p = 0.017 | r = 0.223, p = 0.345 |
| CP (u/L) versus Copper (µg/L) | r = 0.518, p = 0.019 | r = 0.229, p = 0.332 |
| CP (u/L) versus AOPP (µmol/L) | r = −0.445, p = 0.049 | r = 0.195, p = 0.411 |
| Vit. E (mg/L) versus Vit. A (mg/L) | r = 0.521, p = 0.019 | r = 0.566, p = 0.009 |
| ∆Vit. E (mg/L) versus ∆Vit. A (mg/L) | r = 0.553, p = 0.011 | r = 0.520, p = 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, L.; Mizera, O.; Gromadzińska, J.; Janasik, B.; Mikołajewska, K.; Mróz, A.; Wąsowicz, W. Changes in Oxidative Stress, Inflammation, and Muscle Damage Markers Following Diet and Beetroot Juice Supplementation in Elite Fencers. Antioxidants 2020, 9, 571. https://doi.org/10.3390/antiox9070571
Kozłowska L, Mizera O, Gromadzińska J, Janasik B, Mikołajewska K, Mróz A, Wąsowicz W. Changes in Oxidative Stress, Inflammation, and Muscle Damage Markers Following Diet and Beetroot Juice Supplementation in Elite Fencers. Antioxidants. 2020; 9(7):571. https://doi.org/10.3390/antiox9070571
Chicago/Turabian StyleKozłowska, Lucyna, Olga Mizera, Jolanta Gromadzińska, Beata Janasik, Karolina Mikołajewska, Anna Mróz, and Wojciech Wąsowicz. 2020. "Changes in Oxidative Stress, Inflammation, and Muscle Damage Markers Following Diet and Beetroot Juice Supplementation in Elite Fencers" Antioxidants 9, no. 7: 571. https://doi.org/10.3390/antiox9070571