Assessment of the In Vivo Antioxidant Activity of an Anthocyanin-Rich Bilberry Extract Using the Caenorhabditis elegans Model
Abstract
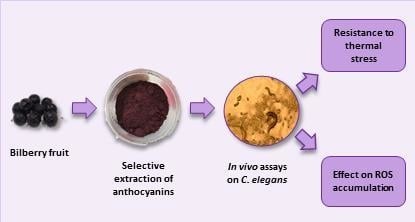
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Bilberry Fruit Samples
2.3. Preparation of the Anthocyanin-Rich Extract from Bilberry Fruit
2.4. HPLC-DAD-MS Analysis of Anthocyanin-Rich Extract
2.5. Strains and Maintenance Conditions
2.6. Stress Assays
2.7. Accumulation of Reactive Oxygen Species (ROS)
2.8. Statistical Analyses
3. Results and Discussion
3.1. Analysis of Anthocyanins in the Bilberry Extract
3.2. C. elegans Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. fruits as a novel source of phenolic compounds with health benefits and industrial applications-a review. Curr. Pharm. Des. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 55–67. [Google Scholar]
- Dried bilberry fruit, Vaccinium myrtillus L. fructus siccus. In Herbal Medicine: Summary for the Public; European Medicines Agency: London, UK, 2015; Available online: http://www.ema.europa.eu/docs/HerbalSummary_of_assessment_report_for_the_public/2015/12/WC500198940.pdf (accessed on 28 July 2017).
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Liu, Y.; Fillmore, S.A.E. Human anthocyanin bioavailability: Effect of intake duration and dosing. Food. Funct. 2017, 8, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Ferrars, R.M.; Cassidy, A.; Curtis, P.; Kay, C.D. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol. Nutr. Food Res. 2014, 58, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Ann. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regener. Res. 2014, 9, 1557–1566. [Google Scholar]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, J.F.; Monteiro, V.V.S.; De Souza Gomes, R.; Do Carmo, M.M.; Da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Kallaur, A.; Simão, A.N.; Morimoto, A.K.; Lopes, J.; Panis, C.; Petenucci, D.L.; Da Silva, E.; Cecchini, R.; Kaimen-Maciel, D.R.; et al. Oxidative stress in multiple sclerosis patients in clinical remission: Association with the expanded disability status scale. J. Neurol. Sci. 2012, 321, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.R. ROS as signalling molecules in T cells evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis. Redox. Rep. 2005, 10, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Freeman, B.A. Reactive oxygen species in human health and disease. Nutrition 2001, 17, 161–165. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 391, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Burdulis, D.; Sarkinas, A.; Jasutienè, I.; Stackivicenè, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta. Pol. Pharm. 2009, 66, 399–408. [Google Scholar] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Halliwell, B. Medicinal plants and antioxidants: What do we learn from cell culture and Caenorhabditis elegans studies? Biochem. Biophys. Res. Commun. 2010, 394, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Watjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Watjen, W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 314–323. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Dueñas, M.; González-Manzano, S.; Cabello, J.; Santos-Buelga, C.; González-Paramás, A.M. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res. Int. 2012, 46, 514–521. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gomez-Orte, E.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- González-Manzano, S.; González-Paramás, A.M.; Delgado, L.; Patianna, S.; Surco-Laos, F.; Dueñas, M.; Santos-Buelga, C. Oxidative status of stressed Caenorhabditis elegans treated with epicatechin. J. Agric. Food Chem. 2012, 60, 8911–8916. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Sánchez-Hernández, E.; Romero, M.R.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Exploring target genes involved in the effect of quercetin on the response to oxidative stress in Caenorhabditis elegans. Antioxidants 2019, 8, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Chen, Y.; Azat, R.; Zheng, X. Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxid. Med. Cell Longev. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Müller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.; Roxo, M.; Krstin, S.; Röhrig, T.; Richling, E.; Wink, M. An anthocyanin-rich extract of açai (Euterpe precatoria mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Tambara, A.L.; de Los Santos Moraes, L.; Dal Forno, A.H.; Boldori, J.R.; Gonçalves Soares, A.T.; de Freitas Rodrigues, C.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C.C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2008, 5, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano-Bailon, M.T.; Santos-Buelga, C. Polyphenol extraction from foods. In Methods in Polyphenol Analysis; Santos-Buelga, C., Williamson, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 1–16. [Google Scholar]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and isolation of phenolic compounds. In Natural Products Isolation. Methods in Molecular Biology, 3rd ed.; Sarker, S., Nahar, L., Eds.; Humana Press: New York, NY, USA, 2012; Volume 864, pp. 427–464. [Google Scholar]
- Poole, C.F. New trends in solid-phase extraction. Trends. Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; González-Manzano, S.; Dueñas, M. Analysis and occurrence of flavonoids in foods and biological samples. In Recent Advances in Medicinal Chemistry; Atta-ur-Rahman, M., Iqbal, C., Perry, G., Eds.; Bentham Science Publishers: Sharjah, UAE, 2015; Volume 2, pp. 10–58. [Google Scholar]
- Espín, S.; González-Manzano, S.; Taco, V.; Poveda, C.; Ayuda-Durán, B.; González-Paramas, A.M.; Santos-Buelga, C. Phenolic composition and antioxidant capacity of yellow and purple-red Ecuadorian cultivars of tree tomato (Solanum betaceum Cav.). Food Chem. 2016, 194, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Hope, I.A. C. elegans. A Practical Approach. In The Practical Approach Series; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Saul, N.; Pietsch, K.; Menzel, R.; Stürzenbaum, S.R.; Steinberg, C.E.W. Catechin induced longevity in C. elegans: From key regulator genes to disposable soma. Mech. Ageing Dev. 2009, 130, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Malakhova, L.V.; Masalimov, Z.K.; Chernikov, A.V. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002, 30, 1354–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies. Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Qiao, X.; Li, X.; Li, Y.; Zhang, Y.; Chen, C. The decay of Redox-stress Response Capacity is a substantive characteristic of aging: Revising the redox theory of aging. Redox Biol. 2017, 11, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Gómez-Orte, E.; Cabello, J.; Santos-Buelga, C. Deglycosylation is a key step in biotransformation and lifespan effects of quercetin-3-O-glucoside in Caenorhabditis elegans. Pharmacol. Res. 2013, 76, 41–48. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Sturzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid-and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saul, N.; Pietsch, K.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Diversity of polyphenol action in Caenorhabditis elegans: Between toxicity and longevity. J. Nat. Prod. 2011, 74, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. NeuroMol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milbury, P.E.; Kalt, W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; White, T.M.; McColl, G.; Jenkins, N.L.; Babich, S.; Candido, E.P.; Johnson, T.E.; Lithgow, G.J. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B281–B287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, A.; Coleen, T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.T.; Hu, P.J. Insulin/Insulin-Like Growth Factor Signaling in C. elegans; WormBook, The C. elegans Research Community, Eds.; WormBook: Pasadena, CA, USA, 2013. [Google Scholar]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88 Pt B, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Peak Number | Compound | mg/g of Extract |
|---|---|---|
| 1 | Delphinidin 3-O-galactoside | 56.8 |
| 2 | Delphinidin 3-O-glucoside | 70.1 |
| 3 | Cyanidin 3-O-galactoside | 27.1 |
| 4 | Delphinidin 3-O-arabinoside | 47.8 |
| 5 | Cyanidin 3-O-glucoside | 37.3 |
| 6 | Petunidin 3-O-galactoside | 22.9 |
| 7 | Cyanidin 3-O-arabinoside | 41.5 |
| 8 | Petunidin 3-O-glucoside | 27.5 |
| 9 | Peonidin 3-O-galactoside + Petunidin 3-O-arabinoside | 17.5 |
| 10 | Peonidin 3-O-glucoside + Malvidin 3-O-galactoside | 34.7 |
| 11 | Malvidin 3-O-glucoside | 65.0 |
| 12 | Malvidin 3-O-arabinoside | 8.8 |
| 13 | Cyanidin | 10.9 |
| 14 | Petunidin | 5.7 |
| 15 | Malvidin | 5.3 |
| Total anthocyanins | 478.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Paramás, A.M.; Brighenti, V.; Bertoni, L.; Marcelloni, L.; Ayuda-Durán, B.; González-Manzano, S.; Pellati, F.; Santos-Buelga, C. Assessment of the In Vivo Antioxidant Activity of an Anthocyanin-Rich Bilberry Extract Using the Caenorhabditis elegans Model. Antioxidants 2020, 9, 509. https://doi.org/10.3390/antiox9060509
González-Paramás AM, Brighenti V, Bertoni L, Marcelloni L, Ayuda-Durán B, González-Manzano S, Pellati F, Santos-Buelga C. Assessment of the In Vivo Antioxidant Activity of an Anthocyanin-Rich Bilberry Extract Using the Caenorhabditis elegans Model. Antioxidants. 2020; 9(6):509. https://doi.org/10.3390/antiox9060509
Chicago/Turabian StyleGonzález-Paramás, Ana M., Virginia Brighenti, Laura Bertoni, Laura Marcelloni, Begoña Ayuda-Durán, Susana González-Manzano, Federica Pellati, and Celestino Santos-Buelga. 2020. "Assessment of the In Vivo Antioxidant Activity of an Anthocyanin-Rich Bilberry Extract Using the Caenorhabditis elegans Model" Antioxidants 9, no. 6: 509. https://doi.org/10.3390/antiox9060509