Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis
Abstract
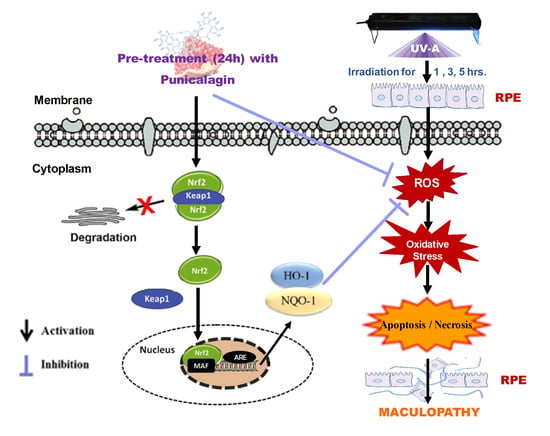
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions and UV Exposure
2.2. Cell Viability
2.3. Detection of ROS
2.4. RNA Isolation and RT-PCR
2.5. Quantification of Intracellular Levels and Nuclear Activation of Nrf2
2.6. Bax and Bcl-2 Detection
2.7. Statistical Analysis
3. Results
3.1. Protective Role of PUN against UV-A-Induced Oxidative Stress and Cell Death
3.2. PUN Protects ARPE-19 Cells from UV Damages by Activating Nrf2/HO-1/NQO1 Signaling
3.3. PUN Modulates UV-A-Induced Bax and Bcl-2 Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauß, O. Pharmacology of the retinal pigment epithelium, the interface between retina and body system. Eur. J. Pharmacol. 2016, 787, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A. Retinal pigment epithelial cells as a therapeutic tool and target against retinopathies. Drug Discov. Today 2018, 23, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.; Krebs, M.P.; Mao, H.; Jones, K.; Conners, M.; Lewin, A.S. Pathological Consequences of Long-Term Mitochondrial Oxidative Stress in the Mouse Retinal Pigment Epithelium. Exp. Eye Res. 2012, 101, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Tringali, G.; Sampaolese, B.; Clementi, M.E. Expression of early and late cellular damage markers by ARPE-19 cells following prolonged treatment with UV-A radiation. Mol. Med. Rep. 2016, 14, 3485–3489. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defence. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The Age-Related Eye Disease 2 Study: Micronutrients in the Treatment of Macular Degeneration. Adv. Nutr. 2017, 8, 40–53. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef]
- Basiri, S. Evaluation of antioxidant and antiradical properties of Pomegranate (Punica granatum L.) seed and defatted seed extracts. J. Food Sci. Technol. 2015, 52, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Mahal, H.S.; Kapoor, S.; Aradhya, S.M. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. Food Chem. 2007, 55, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tuuli, M.G.; Longtine, M.S.; Shin, J.S.; Lawrence, R.; Inder, T.; Michael Nelson, D. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1142–E1152. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Hsu, Y.F.; Lin, T.C.; Hsu, H.Y. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytother. Res. 2001, 15, 206–212. [Google Scholar] [CrossRef]
- Clementi, M.E.; Pani, G.; Sampaolese, B.; Tringali, G. Punicalagin reduces H2O2-induced cytotoxicity and apoptosis in PC12 cells by modulating the levels of reactive oxygen species. Nutr. Neurosci. 2018, 21, 447–454. [Google Scholar] [CrossRef]
- Saito, Y.; Kuse, Y.; Inoue, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Transient acceleration of autophagic degradation by pharmacological Nrf2 activation is important for retinal pigment epithelium cell survival. Redox Biol. 2018, 19, 354–363. [Google Scholar] [CrossRef]
- Lambros, M.L.; Plafker, S.M. Oxidative Stress and the Nrf2 Anti-Oxidant Transcription Factor in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar]
- Kleszczyński, K.; Zillikens, D.; Fischer, T.W. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar]
- Bucolo, C.; Drago, F.; Maisto, R.; Romano, G.L.; D’Agata, V.; Maugeri, G.; Giunta, S. Curcumin Prevents High Glucose Damage in Retinal Pigment Epithelial Cells Through ERK1/2-mediated Activation of the Nrf2/HO-1 Pathway. J. Cell. Physiol. 2019, 234, 17295–17304. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, S.; Yin, P.; Li, D.; Mei, C.; Yu, X.; Shi, Y.; Jiang, L.; Liu, F. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int. J. Hyperth. 2016, 32, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, Y.; Zhao, J.; He, S.; Wang, Y.; Lin, Y.; Wang, N.; Liu, Q. Punicalagin, a PTP1B inhibitor, induces M2c phenotype polarization via up-regulation of HO-1 in murine macrophages. Free Radic. Biol. Med. 2017, 110, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, C.Y.; Hwang, J.; Jo, K.; Kim, S.; Suh, H.J.; Choi, H.S. Punicalagin, a Pomegranate-Derived Ellagitannin, Suppresses Obesity and Obesity-Induced Inflammatory Responses Via the Nrf2/Keap1 Signaling Pathway. Mol. Nutr. Food Res. 2019, 63, e1900574. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Peng, X.; Wei, Z.F.; Wei, F.Y.; Wang, W.; Ma, W.D.; Yao, L.P.; Fu, Y.J.; Zu, Y.G. Geraniin Exerts Cytoprotective Effect Against Cellular Oxidative Stress by Upregulation of Nrf2-mediated Antioxidant Enzyme Expression via PI3K/AKT and ERK1/2 Pathway. Biochim. Biophys. Acta 2015, 1850, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. A new possible mechanism by which punicalagin protects against liver injury induced by type 2 diabetes mellitus: Upregulation of autophagy via the Akt/FoxO3a signaling pathway. J. Agric. Food Chem. 2019, 67, 13948–13959. [Google Scholar] [CrossRef]
- Cheng, X.; Yao, X.; Xu, S.; Pan, J.; Yu, H.; Bao, J.; Guan, H.; Lu, R.; Zahng, L. Punicalagin induces senescent growth arrest in human papillary thyroid carcinoma BCPAP cells via NF-κB signaling pathway. Biomed. Pharmacother. 2018, 103, 490–498. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chang, S.F.; Chau, S.F.; Chiu, S.C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules 2019, 9, 380. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.W.; Chen, C.; Wang, L.; Li, Y.D.; Hu, Z.L. S-allyl cysteine protects retinal pigment epithelium cells from hydroquinone-induced apoptosis through mitigating cellular response to oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2120–2128. [Google Scholar]
- Zhao, B.; Wang, Z.; Han, J.; Wei, G.; Yi, B.; Li, Z. Rhizoma Paridis total saponins alleviate H2O2 induced oxidative stress injury by upregulating the Nrf2 pathway. Mol. Med. Rep. 2020, 21, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrrow, J.R.; Hicks and Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2015, 24 Pt B, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.H. Involvement of Nrf2 in Ocular Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Li, J.; Johnson, D.A.; Stein, T.D.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Johnson, J.A. Nrf2, a multi-organ protector? FASEB J. 2005, 19, 1061–1066. [Google Scholar] [CrossRef] [Green Version]
- Niture, S.K.; Jaiswal, A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [Green Version]
- Rao, F.; Tian, H.; Li, W.; Hung, H.; Sun, F. Potential role of punicalagin against oxidative stress induced testicular damage. Asian J. Androl. 2016, 18, 627–632. [Google Scholar]
- Xu, X.; Li, H.; Hou, X.; Li, D.; He, S.; Wan, C.; Yin, P.; Liu, M.; Liu, F.; Xu, J. Punicalagin Induces Nrf2/HO-1 Expression via Upregulation of PI3K/AKT Pathway and Inhibits LPS-Induced Oxidative Stress in RAW264.7 Macrophages. Mediat. Inflamm. 2015, 2015, 380218. [Google Scholar]
- Zhao, C.R.; Gao, Z.H.; Qu, X.J. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010, 34, 523–533. [Google Scholar] [CrossRef] [PubMed]





| Genes | Forward | Reverse | Product Length |
|---|---|---|---|
| Nrf2 (NM_006164.5) | GTCACATCGAGAGCCCAGTC | ACCATGGTAGTCTCAACCAGC | 193 |
| HO-1 (NM_002133.3) | CTGGAGGAGGAGATTGAGCG | ATGGCTGGTGTGTAGGGGAT | 152 |
| NQO1 (NM_000903.3) | GGTTTGAGCGAGTGTTCATAGG | GCAGAGAGTACATGGAGCCAC | 129 |
| GAPDH (NM_002046.7) | AACGGATTTGGTCGTATTG | GGAAGATGGTGATGGGATT | 208 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clementi, M.E.; Sampaolese, B.; Sciandra, F.; Tringali, G. Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis. Antioxidants 2020, 9, 473. https://doi.org/10.3390/antiox9060473
Clementi ME, Sampaolese B, Sciandra F, Tringali G. Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis. Antioxidants. 2020; 9(6):473. https://doi.org/10.3390/antiox9060473
Chicago/Turabian StyleClementi, Maria Elisabetta, Beatrice Sampaolese, Francesca Sciandra, and Giuseppe Tringali. 2020. "Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis" Antioxidants 9, no. 6: 473. https://doi.org/10.3390/antiox9060473